en
names in breadcrumbs


Eastern Poison-ivy (Toxicodendron radicans) is found throughout the eastern United States and adjacent Canada south to Guatemala. Poison-ivy and its close relatives are well-known for possessing skin-irritating oil (urushiol), which can cause severe allergic reactions in humans.
The taxonomy and nomenclature of North American Toxicodendron has been in flux for over a century, largely due to confusing within-species variation in growth form, leaf and leaflet shape, and other features (e.g., Gillis 1971; Gartner 1991). This has resulted in an abundance of synonyms, but five species are now generally recognized: Common Poison-ivy (T. radicans), Western Poison-ivy (T. rydbergii), Eastern Poison-oak (T. pubescens), Western Poison-oak (T. diversilobum), and Poison-sumac (T. vernix) (Senchina 2006). Eastern Poison-ivy is the only Toxicodendron species in eastern North America that is typically a climbing (or straggling) vine with aerial roots (Western Poison-ivy, Eastern Poison-oak, and Poison-sumac do not climb but are instead erect or suberect shrubs) (Gleason and Cronquist 1991). Older plants often develop thick, reddish brown stems that ascend tree trunks. For reasons that are not yet clear, Eastern Poison-ivy grows more abundantly on some tree species than on others (Talley et al. 1996). Eastern and Western Poison-ivy may intergrade to some extent, making identification less clear in some areas.
Toxicodendron radicans is supposedly also present in eastern Asia, with the North American form known as T. radicans radicans and the Asian form known as T. radicans hispidum. However, molecular phylogenetic analyses by Nie et al. (2009) suggest that these two taxa are not each other's closest relatives and that the Asian form is, instead, the sister group to a clade including all four trifoliate (three-leafleted) North American Toxicodendron (Poison-sumac has 7 to 13 leaflets per leaf and its apparent closest relative lives in eastern Asia). This would imply that what is currently known as T. r. hispidum should be treated as a distinct species.
Senchina (2008) reviewed the literature on animal and fungal associates of Toxicodendron in North America with a particular eye toward identifying potential biological control agents. Interest in finding new ways to control poison-ivy and its relatives may increase in coming years given data suggesting that these plants may become more abundant and more ‘‘toxic’’ in the future, potentially affecting global forest dynamics and human health (Mohan et al. 2006).
(Gillis 1971; Nie et al. 2009 and references therein)
Poison ivy is a ubiquitous species that thrives in a variety of habitats from woodlands, to fields, beaches, and gardens and on trees, telephone poles, signs, and fences. The plant has no strong soil preferences and, as long as there is sufficient water and sun, it will grow and flourish. Poison ivy is adaptable and variable, as it is pervasive. This plant shows a variety of growth habits depending on its environmental circumstances. It can appear as a low-growing herb, a vine that creeps along the ground, or a thick, hairy woody plant climbing up the trunk of a tree. Its leaves can vary from smooth and round to narrow and sharp. The leaves are often shiny reddish in color when they are young and in fall they turn yellow or deep red like other fall foliage.
This description covers characteristics that may be relevant to fire ecology and is not meant for identification. Keys for identification are available (e.g., [47,87,88,92,109,204,223,266,268,281]).
Form and architecture: Eastern poison-ivy is a rhizomatous plant that may be an erect, low shrub or a high-climbing or trailing liana that climbs via aerial roots [40,87,92,124,137,204,234,244,283]. Western poison-ivy is a rhizomatous, erect, low shrub that has no aerial roots, so it does not climb [47,87,92,109,133,156,214,234,266,268]. Eastern poison-ivy plants are much branched and woody throughout (see Image 1), whereas western poison-ivy plants are sparsely branched and woody only about 2 to 48 inches (5-122 cm) from the base (see Image 2) [47,87,92,156,214,223,266,275,275]. The stems of poison-ivies are thin-barked [32]. When a shrub, eastern poison-ivy may be 2 to 10 feet (0.5-3.0 m) tall [60,283]. When climbing, the main stem may be >160 feet (50 m) long [240,283] and up to 6 inches (15 cm) in diameter [60,88,234,240,266,283]. Western poison-ivy is typically <3 feet (1 m) tall but may be up to 10 feet (3 m) tall [47,83,87,92,109,214,234,245]. In Utah, western poison-ivy plants are rarely >1 foot (0.3 m) tall [275]. Aboveground stems of both species arise from much-branched rhizomes [83].
Leaves: Poison-ivies have alternate, deciduous, compound leaves. Each leaf has 3 leaflets [47,87,202,204,214,234,244,275]. Leaflets of both species vary greatly in shape and size [88,266]. Eastern poison-ivy leaflets are 2 to 8 inches (5-20 cm) long and 1 to 6 inches (2-16 cm) wide [87,88,204,240,244,283]. Western poison-ivy leaflets are 1 to 6 inches (2-15 cm) long and 1 to 4 inches (2-10 cm) wide [47,234,266,275].
Reproductive structures: Poison-ivy flowers have 5 petals and occur in loose clusters from the axils of the leaves [234,266]. The inflorescence of eastern poison-ivy usually has more than 25 flowers, whereas the inflorescence of western poison-ivy usually has fewer than 25 flowers [47,83,87]. Poison-ivy fruit is a small, dry, round drupe [87,92,202,266,275]. Drupes occur in dense, erect or ascending "grape-like" clusters [109,240]. Each drupe is 3 to 7 mm in diameter [47,87,88,92,204,234,240,245,266,275] and has a single, hard, 3- to 4-mm diameter seed [240,245].
Rhizomes and roots: Poison-ivies have creeping, underground rhizomes [47,87,92,109,156,245,283]. Rhizomes may be long. They may occur at the surface or be deep in the soil [144], although they tend to be shallow, extending only about 4 to 6 inches (10-15 cm) deep [108]. Poison-ivies have fibrous roots that grow from the rhizomes; the roots may be up to 12 feet (3.7 m) deep [188,261].
Eastern poison-ivy has abundant adventitious, aerial roots that adhere to supports [87,124,204,244,266]. Aerial roots are produced when a stem contacts a substrate [124,283].
Stand and age class structure: As a result of an extensive network of rhizomes, poison-ivies frequently form dense thickets [83,92,92,124,223,234]. These thickets may represent a single clone or several individuals [47,83,87]. Eastern poison-ivy density ranged from <0.1 stem/ha in white oak-red maple stands in northeastern Pennsylvania [57] to more than 290,000 stems/ha in logged loblolly pine stands in Texas [171]. In sandhills of northeastern Colorado, western poison-ivy occurred in patches from many square feet up to 1 acre (0.4 ha) in sandhill muhly (Muhlenbergia pungens) communities, especially on eastern slopes [206]. Poison-ivies may inhibit the growth of other vegetation [68]. See Impacts for more information.
Dense eastern poison-ivy in a clearcut on the Bessey Ranger District, Nebraska. Photo courtesy of the USDA Forest Service-Region 2-Rocky Mountain Region Archive, Bugwood.org.The longevity of poison-ivy plants was unknown as of this writing (2012). Gillis [84] reported that a 4.7-inch (12 cm) diameter eastern poison-ivy stem had 38 growth rings.
Eastern and western poison-ivy's geographic ranges overlap in the Midwest and Northeast. Together, they are native to every state except California, Alaska, and Hawaii and to every province except Nunavut, the Northwest Territories, and Newfoundland and Labrador. Eastern poison-ivy also occurs on Bermuda and the western Bahamas and in Mexico, Central America, Japan, China, Taiwan, and Russia [47,82,87,88]. It has been introduced in Africa, Europe, New Zealand, and Australia [83,166,283]. In the West, western poison-ivy occurs as far west as the eastern side of the Cascade Range in Washington, Oregon, and southern British Columbia. In the East, disjunct western poison-ivy populations occur at high elevations in the southern Appalachian Mountains in Pennsylvania, West Virginia, and Virginia [47,81,87,223,266].
Eastern poison-ivy:
States and provinces (as of 2012 [263]):
United States: AL, AR, AZ, CT, DC, DE, FL, GA, IA, IL, IN, KS, KY, LA, MA, MD, ME, MI, MN, MO, MS, NC, NE, NH, NJ, NY, OH, OK, PA, RI, SC, SD, TN, TX, VA, VT, WI, WV
Canada: NB, NS, ON, QC
Mexico [83,88,133,202,266,278]
Western poison-ivy:
States and provinces (as of 2012 [263]):
United States: AZ, CO, CT, IA, ID, IL, IN, KS, MA, MD, ME, MI, MN, MT, NC, ND, NE, NH, NM, NV, NY, OH, OK, OR, PA, RI, SD, TX, UT, VA, VT, WA, WI, WV, WY
Canada: AB, BC, MB, NB, NS, ON, PE, QC, SK, YT
To date (2012) there were few published recommendations for managing poison-ivies with fire. Because burning live or dead eastern or western poison-ivy plants creates contaminated smoke that, if inhaled, can cause fever, extreme respiratory problems, or even death [108], prescribed fire should be used cautiously in communities with these species. Nonetheless, prescribed fire is frequently applied in communities with poison-ivies (e.g., [3,10,11,68,119,122,126,142,212,277]).
Poison-ivies often occur in canopy openings (see Successional Status), so small, patchy fires may benefit poison-ivies by providing openings that allow them to spread vegetatively or establish from on- or off-site seed sources. Although poison-ivies often increase after prescribed fire, they may also decrease after fire, depending in part of fire timing, frequency, and severity (see Plant response to fire). Viable poison-ivy seeds have been found in the soil seed bank of some forest communities after fire (see IMMEDIATE FIRE EFFECT ON PLANT) [222]. Thus, poison-ivies may establish from the seed bank after prescribed fire. Prescribed fire may temporarily reduce poison-ivy seed production. However, on the Noxubee National Wildlife Refuge, eastern poison-ivy fruit was abundant 4 years after plants were top-killed by a March prescribed fire in loblolly pine-oak forest. The forest had been burned 7 other times during the previous 12 years [126].
Plant response to fire: Poison-ivies sprout from the root crown and/or from rhizomes after top-kill by fire [68]. Poison-ivies may also establish from the soil seed bank. A study in Arkansas found that at least some eastern poison-ivy seeds in the soil survived a prescribed fire [222], indicating the potential for on-site postfire establishment. Poison-ivies may also establish from off-site animal- or water-dispersed seed after fire. Eastern poison-ivy may establish from seed dispersed from lianas in tree crowns.
Occurrence of poison-ivies after wild and prescribed fires has been reported in a variety of plant communities. Poison-ivies have variable responses to fire; patterns with regard to season, frequency, and severity of fire are not evident, so studies are listed below according to fire response.
Many studies reported that poison-ivies increased soon after fire or within the first growing season [10,11,39,68,110,126]:
Table 3. Studies reporting increases in abundance of poison-ivies soon after fire Species Location Plant Community Fire description Observations eastern poison-ivy south-central Tennessee oak-hickory forest July prescribed fire increased on burned plots and decreased on control plots 1 year after fire compared to prefire levels (Table 5) [68] east-central Mississippi loblolly pine-oak forest early spring prescribed fire; forest had been burned 7 other times during the previous 12 years increased after fire and was most abundant during postfire year 4, when the study ended [126] north-central Florida longleaf pine-turkey oak (Pinus palustris-Quercus laevis) forest January prescribed fire "far greater" in abundance in burned areas than in unburned areas 3 months after fire [11] western poison-ivy southeastern Ontario xeric northern whitecedar-quaking aspen-balsam fir woodland severe June wildfire cover averaged 5.3% and frequency averaged 13.6% 100 days after fire [39] poison-ivies southwestern Illinois post oak/little bluestem barrens low-severity, March prescribed fire absent before fire but present 7 months later [110] northeastern Illinois closed-canopy oak woodland fall prescribed fire greater importance the 1st growing season after fire than before fire [10]Some studies reported that poison-ivies decreased after fire, exhibited little or no change after fire, or that fire had mixed effects on poison-ivies [3,122,142,185,186,212,251,267]:
Table 4. Studies reporting that poison-ivies decreased after fire, exhibited little or no change after fire, or that fire had mixed effects on poison-ivies Species Location Plant Community Fire description Observations eastern poison-ivy northeastern Illinois white oak savanna spring and fall prescribed fires applied 1 to 4 times in 7 years 7 years after fires, density was >3 times lower than before fires; at a nearby unburned control site, density was >4 times lower [142] central North Carolina loblolly pine forest mixed-severity wildfire density was higher on burned than unburned plots 9 years after fire but mean frequency was similar between treatments [186]; 20 years after fire, density had declined by ≥50% [185] poison-ivies northwestern Wisconsin upland jack pine (Pinus banksiana)-northern pin oak savanna March and April prescribed fires; sites were burned 1-4 times in approximately 13 years frequency was similar on burned and unburned control sites sampled 2-17 months after fires [267] Illinois oak-hickory barrens 2 mixed-severity prescribed fires; the 1st fire was applied in late November, the 2nd fire in mid-March 5 years later cover of poison-ivies was similar before and 1 growing season after fires [251] east-central Illinois 20-year-old herb-dominated old field with scattered trees and shrubs 2 successive March prescribed fires; the 1st fire was high-severity, the 2nd fire was low-severity frequency was similar before and 2 years after fires [122] Oklahoma indiangrass tallgrass prairie a summer prescribed fire and a "hotter" March prescribed fire not detected 1 year after fires [3] east-central Texas shortleaf pine-sweetgum-loblolly pine forest low-severity, February and March prescribed fires cover similar before and 1 year after fires [212]Although poison-ivies often increase after fire, the effect may be short term. In a southern Appalachian Virginia pine-shortleaf pine-scarlet oak (Quercus coccinea)-white oak forest, eastern poison-ivy occurred with 5.0% frequency and 0.2% cover 1 growing season after a March prescribed fire. The 2nd growing season, both measures had declined [65]. Two studies in the Black Hills, South Dakota, found that western poison-ivy increased after fire in the short term. The 1st growing season after an April low-severity (<5% top-kill of trees) prescribed fire in bur oak woodlands, western poison-ivy density was higher in burned (3.1 stems/m²) than unburned (0.8 stem/m²) plots (P=0.05). The 2nd growing season after fire, however, its density was similar in burned and unburned plots, which were both similar to prefire levels [227]. In a ponderosa pine-grassland ecotone, western poison-ivy densities were unchanged during the first 2 years after low-severity, April and May prescribed fires [26].
Other researchers reported increased abundance of poison-ivies after fire in the long term. In a 53-year study of postfire forest succession in northern Michigan, poison-ivies had the greatest frequency 38 and 51 years after fire [217].
The effect of fire on poison-ivies depends in part on the plant communities involved. To reduce fuels after Hurricane Hugo, prescribed fire was applied to loblolly pine stands and nonnative Chinese tallow (Triadica sebifera) stands. Burning was conducted in winter and was of low severity, with most of the duff layer remaining unburned. Two years after the prescribed fire, eastern poison-ivy density in loblolly pine stands was similar in burned (22,500 stems/ha) and unburned (25,000 stems/ha) stands. In Chinese tallow stands, however, eastern poison-ivy density was much higher in burned (30,001 stems/ha) than unburned (4,169 stems/ha) stands [231].
Prescribed fire is often used in conjunction with logging. Several studies reported greater abundance of poison-ivies on logged and burned sites than unburned (logged or unlogged) sites [33,125,226], but other studies reported lesser abundance on logged and burned sites than other sites [160,199]. On the Bankhead National Forest, Alabama, liana and vine cover—mostly eastern poison-ivy—on logged stands burned under prescription in fall or spring was twice (range: 11-15%) that of a logged but unburned stand (6%). The plant community was an upland oak-mixed hardwood forest [125]. At the Fort Benning Military Base, Georgia, midstories of longleaf pine-loblolly pine stands were masticated and burned under prescription in either December, May, or July. Eastern poison-ivy abundance in postfire year 1 was greater on treated than on control plots [33]. In southeastern Ontario, frequency of poison-ivies decreased on logged eastern white pine-paper birch-red pine forests 1 month after July prescribed fire, but it also increased on uncut and unburned controls. However, the difference was not significant, and the author concluded that logging and prescribed fire had no effect on frequency or biomass of poison-ivies [226]. In southeastern Virginia and northeastern North Carolina, eastern poison-ivy occurred in neither in an Atlantic white-cedar (Chamaecyparis thyoides) peat swamp that was burned in a wildfire 2 years previously nor an area that was logged and then burned. It was abundant, however, in an untreated area [160]. Three years after treatment in Clemson Experimental Forest, South Carolina, eastern poison-ivy increased on thinned plots and plots burned in a moderately severe April prescribed fire, but it declined on plots that were thinned and burned in a low-severity fire 1 year later. The authors suggested that eastern poison-ivy declined on the thinned and burned plots because of the repeated disturbance [199].
Fire timing, frequency, and severity may affect the response of poison-ivies to fire.
Fire timing: Fire season may affect poison-ivies' response to fire. The number of eastern poison-ivy growing points (defined as any foliage- and root-bearing horizontal stem section >3 inches (8 cm) long) in oak-hickory forest increased more on fall-burned plots than winter-burned plots 1 year after prescribed fires in Chickamauga and Chattanooga National Military Park. The authors suggested that a decrease in the number of eastern poison-ivy growing points on control plots was probably due to summer drought, while the increase in eastern poison-ivy growing points on burned plots was the result of postfire sprouting from surviving stems and underground reproductive structures. Although growing points increased, total biomass was apparently reduced [68].
Table 5. Mean number of eastern poison-ivy growing points/m² in a control and 2 treatments in Chickamauga and Chattanooga National Military Park, Tennessee [68] Treatment Count % change* Pretreatment Posttreatment Control 10.6 5.3 -49.8 a Fall prescribed fire 26.7 38 +42.6 b Winter prescribed fire 12.0 12.5 +4.6 c *Values having different postscripts were significantly different at P=0.05.Fire frequency: Poison-ivies occur on sites where fire is frequent and on sites where fire is infrequent. For example, in logged loblolly pine-shortleaf pine stands in southeastern Arkansas, cover of eastern poison-ivy was similar in stands with a variety of burn histories: 1 year after a fourth 3-year burn cycle (2.3% cover); 4 years after a second 6-year burn cycle (3.4% cover); 1 year after a second 9-year burn cycle (1.3% cover); and in an untreated control stand (1.2% cover). Stands were burned under prescription in December, January, or February [37]. Eastern poison-ivy was common in shortleaf pine stands in Arkansas that had been burned 1 to 5 times in 15 years [236,237]. Poison-ivies occurred in white oak-post oak barrens in east-central Illinois, 1 and 2 years after fall prescribed fire, and at a site burned under prescription 3 times in 10 years [159]. In Missouri, eastern poison-ivy was present in oak-hickory flatwoods burned annually in spring for 8 years [192].
Poison-ivies are often abundant after repeated annual or biennial fires. In a northern pin oak community in east-central Minnesota, poison-ivies were more frequent (12.7%) on a site that was burned prior to leaf-out every spring for 13 years than on an adjacent unburned control site (5.0%) [277]. Low-severity May prescribed fires were applied to 2 eastern white pine-red pine stands in Michigan. Cover of poison-ivies was greater on a stand 2 years after a single fire (1.4%) and a stand 1 year after a second biennial fire (1.3%) than on unburned control plots (0.7%) [180]. For more information, see the Research Project Summary of this study.
Poison-ivies are often common in communities burned at >5-year intervals. At the Teft Savanna Nature Preserve in Indiana, density of poison-ivies in white oak barrens did not change during 20 years despite mixed-severity spring prescribed fire used at an average of every 5.7 years [99]. In a southern Appalachian oak-hickory forest, Holzmueller and others [119] compared understory diversity and composition among oak-hickory stands in the Great Smoky Mountains that were burned 1 to 3 times during 20 years. Poison-ivies were most indicative of stands that were burned 2 times in 20 years (P=0.04) [119]. In Wisconsin, frequency of poison-ivies in a northern pin oak savanna subjected to 2 prescribed fires in 15 years was higher than in adjacent unburned savanna; they apparently established in burned sites after fire opened the canopy [20]. In northern Florida, 2nd-growth loblolly pine-shortleaf pine woodlands were burned under prescription during 75 years at frequencies ranging from 1 to 13 times in 13 years. However, eastern poison-ivy was less abundant on these burned plots than on plots that had not been burned during the 75 years (P<0.02) [163]. At the Cedar Creek Ecosystem Science Reserve, western poison-ivy was a dominant plant in a pin oak-bur oak/big bluestem savanna burned 3 times during 37 years [54].
Other researchers reported that abundance of poison-ivies was reduced on sites burned frequently (every 1 to 4 years) [113] or that poison-ivies were less abundant on sites burned frequently than on sites burned at intervals of 5 years or more [111,141,163]. In DuPage County, Illinois, mean cover of poison-ivies was lower in a northern red oak-white oak-bur oak forest subjected to annual low- to moderate-severity prescribed fire during fall or spring for 17 years (0.05%) than in unburned controls (1.8%) (see the Research Paper by Bowles and others) [31]. Annual spring prescribed fire for 3 years in a big bluestem-prairie dropseed prairie in southwestern Minnesota reduced western poison-ivy cover in the short term. Prior to the fires, western poison-ivy cover was 1.6%. The summer immediately following the 1st prescribed fire, its cover was 0.1%. The following spring, its cover increased slightly to 0.4%. After 2 annual prescribed fires, its cover was less than half of prefire levels (0.7%) [21]. In Escambia Experimental Forest in Alabama, eastern poison-ivy was absent from longleaf pine stands burned biennially in winter, spring, or summer during 22 years, but it had 1.2% cover in unburned control stands [141]. Poison-ivies decreased 81% in a tallgrass prairie in southeastern Michigan that was burned at 1- to 3-year-intervals in April or November over 16 years [113]. In big bluestem-indiangrass-little bluestem prairie in northeastern Nebraska, poison-ivies were absent on sites either burned under prescription annually in April or burned every 4 years in April. However, they were frequent (33%) on sites burned once in 18 years. Prior to the study, all sites were burned every 2 to 3 years, and poison-ivies were absent from all sites [111]. In a south-central Illinois oak-hickory forest, the number of plants of poison-ivies declined 31% after 4 prescribed fires during 8 years. Fires occurred in February and March [252].
Fire severity: Poison-ivies are reported after fire severities ranging from low to high. In Ontario, they were dominant understory plants in a quaking aspen stand 20 years after a severe wildfire [273]. In a Rocky Mountain juniper community in the Little Missouri Badlands, North Dakota, that sustained different levels of fire damage, western poison-ivy had the highest frequency (33%) in stands with upper crown damage. It had 20% frequency in stands with surface damage and in stands with lower crown damage [205].
Eastern poison-ivy may form fuel ladders. Fuel ladders formed by climbing eastern poison-ivy stems were reduced 1 year after a July prescribed fire in Chickamauga and Chattanooga National Military Park compared to prefire levels. Although all fine fuels present in these ladders were consumed by flare-ups, some large main stems remained. However, nearly all of the remaining stems were dead because the fire consumed them up to about 3 feet (1 m) from the ground, thus severing them from their roots. The authors concluded that because large ladder fuels were separated from the ground, the prescribed fire reduced the possibility of future surface fires becoming crown fires [68].
Although other fuel characteristics for poison-ivies were unknown as of this writing (2012), they are likely to differ substantially between eastern poison-ivy, which climbs and is woody throughout, and western poison-ivy, which does not climb and is woody only about 2 to 48 inches (5-122 cm) from the base (see Botanical description).
Poison-ivy seeds have a dormant embryo and a hard endocarp that inhibits germination. In a series of germination tests of eastern poison-ivy seeds, only seeds from Florida and Bermuda germinated without cold stratification to break dormancy [84].
Seed viability and germination rates may be high. In sand dunes along Lake Erie, all fruits collected from poison-ivies had viable seeds [285]. Mean germination reported in the literature ranged from 0% to 96% [84,139,218]. Seeds of poison-ivies remain viable for at least 6 years in the laboratory [84].
Typically, seeds of poison-ivies must be scarified and/or cold-stratified for long periods (3-4 months) for germination to occur [218]. In the laboratory, ≤1% of seeds of poison-ivies collected in December and February and planted in the laboratory germinated without scarification or stratification. Seeds scarified with sulfuric acid had better germination (63%) than unscarified seeds. Seeds collected in February and stratified had the best germination (90%-96%). The authors suggested that seeds picked in February had the highest germination rates because they experienced a longer period of cold stratification in the field prior to collection compared to seeds picked in December. Scarification of seeds prior to stratification did not improve germination (66%-72%) [218]. Germination of stratified eastern poison-ivy seeds from 14 locations throughout the species' range varied from 4% to 82%. Germination was 0% to 3% for western poison-ivy seeds from 5 locations. It was unclear why rates of western poison-ivy seed germination were so much lower than those of eastern poison-ivy [84].
In nature, poison-ivy seeds may be scarified by passing through an animal's digestive tract [171]. Western poison-ivy seeds that had passed through sharp-tailed grouse digestive tracts gave 86% germination after warm, then cold stratification [139]. However, no information on controls was provided in this study. In a bur oak-western poison-ivy grove in Manitoba, western poison-ivy seeds extracted from ruffed grouse feces and those taken directly from plants in February had similar germination rates [194].
Germination of poison-ivies in the wild may be high. In South Carolina, seedfall of eastern poison-ivy was positively associated with the number of germinants during 2 years (P<0.01), indicating that seedfall from the previous year strongly contributed to that germination [131].
The aboveground portions of poison-ivies are probably easily killed by fire. According to Bragg [32], the thin-barked stems are easily killed by even low-severity fire. However, poison-ivies may sprout from rhizomes or root crowns that are protected from fire by soil. Some studies reported sprouting of poison-ivies after fire (e.g., [68,126]). Most poison-ivy rhizomes extend only about 4 to 6 inches (10-15 cm) deep [108]. However, even the most severe fires rarely damage plant tissues below 2 inches (10 cm) in the soil [232]. Thus, many rhizomes are probably insulated from heat damage by soil and likely survive most fires. In Chickamauga and Chattanooga National Military Park, Tennessee, some aboveground horizontal stems of eastern poison-ivy survived a July prescribed fire. Many surviving aboveground horizontal stems sprouted after the fire, although a majority of postfire growth appeared to be from belowground reproductive structures [68].
The effect of fire on poison-ivy seeds in the seed bank was unclear as of this writing (2012). Prescribed fire was applied to 2 oak stands near Russellville, Arkansas, in February, and seeds were germinated in a greenhouse from soils taken from the upper 2 inches (5.0 cm) of soil immediately prior to and after the fire. In soils from one stand, the density of eastern poison-ivy germinants was 76% less in burned than in unburned soils. In the other stand, the density of germinants was 300% more in burned than in unburned soils [222].
Poison-ivies provide food for birds, small mammals, wild ungulates, and livestock.
Wildlife: At least 75 species of birds, particularly gallinaceous birds such as wild turkeys, northern bobwhites, ruffed grouse, and sharp-tailed grouse, eat the fruits and seeds of poison-ivies (e.g., [17,109,124,139,147,171,187,245,279]). Many mammals—including bears, mule deer, white-tailed deer, moose, foxes, woodchucks, muskrats, rabbits, squirrels, woodrats, and mice—consume the leaves, stems, and fruits of poison-ivies (e.g., [97,124,181,187,194,209,249,255]). In southern Indiana, eastern poison-ivy was 1 of the 7 most important plants consumed year-round by white-tailed deer. White-tailed deer ate the leaves with greater frequency in summer (81%) than in spring (67%) [235]. Poison-ivy fruits may be particularly important during winter [17,81] or during poor mast years [61,97] when other fruits are unavailable. In Maryland, ripe eastern poison-ivy fruits were available on plants in September, but birds did not begin harvesting them until late October [52].
Livestock: According to a fact sheet, livestock typically browse poison-ivies only sparsely [262]. However, heavy livestock browsing of poison-ivies may occur and sometimes reduces abundance of poison-ivies locally. See Control for more information on this topic.
Palatability and nutritional value: Wildlife and livestock can browse poison-ivies without ill effects to the animals [42,109,171,262]. Western poison-ivy palatability is generally low to good. It is "poor" to "good" forage for small mammals and birds; poor forage for pronghorn, elk, mule deer, and white-tailed deer; and poor to fair forage for cattle, domestic sheep, and horses. It is rated poor in both energy and protein value [55].
Cover value: According to Dittberner and Olson [55], western poison-ivy cover value for wildlife is "poor" to "fair". However, western poison-ivy was the most common understory plant in a riparian eastern cottonwood/willow community in Scotts Bluff National Monument, Nebraska, and this community provided important cover for more than 80 species of birds, mammals, reptiles, and amphibians [45].
In spring, eastern poison-ivy leaves appear on vertical stems that have overwintered [171]. In northeastern Colorado, new shoots appeared on western poison-ivy in mid-May [206]. In Canada, overwintering vertical stems of poison-ivies start growing in late May or early June. Horizontal "rootstocks" and new vertical shoots from the rootstocks are first produced in late June and reach their maximum development during July and August [171]. In Georgia, leaves were fully expanded in early March [76]. In Tennessee, eastern poison-ivy leafed out as early as mid-March, although leaf-out occurred in mid-April the spring after a July prescribed fire [68].
The timing of flowering and fruiting is related to latitude, with flowering dates progressively later toward the north [84]. In Canada, flower buds of poison-ivies, which form on new growth in late summer and early fall of the previous year, open in late May or early June [171]. According to Lakela [144], flowering occurs when leaves are about half open [144]. Mulligan and Junkins [171] stated that flowering does not occur until leaves are fully expanded. Generally, eastern poison-ivy flowers from March to July [40,168,244,281] and fruits from July to January [124,156,204,229,234,283]. Western poison-ivy flowers from April to June [19,47,55,92,133,245] and fruits from July to November (Table 1) [156,234]. On the Great Plains, western poison-ivy plants sometimes bloom twice in one season; first in May or June and again in August or September [92]. Eastern poison-ivy plants in southeastern Arizona may have 2 periods of maximum flowering, 1 in April and May and 1 in mid-August to early September [84]. In Canada, peak flowering of poison-ivies occurs in June, but some additional flowering occurs sporadically until early fall [171]. The length of time required for fruits to ripen varies by site. In southern Florida, eastern poison-ivy fruits ripen in 3 months; in northern Florida fruits ripen in 4 months; and in southern New England fruits ripen in 2 months [84]. Poison-ivy fruits often persist over winter and into early spring [92,140,171,234,240].
Table 1. General flowering dates for poison-ivies throughout their ranges in North America Species Location Flowering period Eastern poison-ivy Arkansas throughout May-July [124] Florida Florida panhandle March-April [40] northern Florida April-May [229] Illinois throughout May-July [168] Mississippi throughout April-October [63] Missouri throughout May-July [283] North and South Carolina throughout late April-May [204] Texas central mid-April to May [55] West Virginia throughout May-July [244] East Gulf and Atlantic coasts from Texas north to southern Nova Scotia March-June [60] Great Plains north-central late May [240] Northeast northeastern and north-central United States and adjacent Canada May-July [87] Southeast Blue Ridge Mountains May-June [281] Southwest southeastern Arizona and the Sierra Madre Occidental, Mexico March-June [83] Ontario throughout June-early July [234] Western poison-ivy Arizona throughout April-September [133] Colorado throughout May-June [55] Kansas throughout May-June [19] North Dakota throughout May-July [55,241] Utah throughout June-July [55] Wyoming throughout June-September [55] Great Plains throughout May-June [92,245], sometimes again August-September [92] Intermountain region throughout May-June [47] Ontario throughout June-early July [234]Poison-ivies' wide distributions suggest they are adapted to a wide range of climates [171]. They grow in semiarid [115,205,261], humid [213,269,272], subtropical, and tropical [269] regions of the United States. In coastal Maine, poison-ivies occurred in a perhumid climate with localized fog [165]. Poison-ivies occur in regions of the United States and Canada with average annual temperatures ranging from 39 °F (4 °C) (southern Quebec) [265] to 72 °F (22 °C) (central Florida) [1] and average annual rainfall ranging from 15.7 inches (400 mm) (southeastern Arizona) [211] to 61.9 inches (1572 mm) (southern Florida) [62]. The average number of frost-free days ranges from 0 days (southern Florida) [62] to 111 days (South Dakota) [205]. Poison-ivies appear intolerant of extreme cold. In Canada, sections of horizontal rootstocks and vertical stems of poison-ivies were often winter-killed [171].
Lianas, such as eastern poison-ivy, are hypothesized to benefit from warmer temperatures and elevated carbon dioxide levels predicted by global climate change [152,221]. Under experimentally elevated levels of atmospheric carbon dioxide, eastern poison-ivy increased photosynthesis, water-use efficiency, growth, and population biomass during 5 growing seasons. Plants exposed to elevated carbon dioxide also produced more urushiol. These results suggested that under elevated levels of carbon dioxide, eastern poison-ivy may grow larger and become more noxious [166]. However, in mixed-hardwood forests in Wisconsin, it decreased in abundance during 45 years despite increased atmospheric carbon dioxide levels and increased mean winter temperatures of 4.3 °F (2.4 °C). This suggested that eastern poison-ivy was limited by factors other than carbon dioxide levels and low winter temperatures, such as light availability [152]. See Schnitzer and others [221] and Mohan and others [167] for more information on possible effects of climate change on eastern poison-ivy.
Plant communities: Poison-ivies occur in a variety of plant communities from barrier-island sand dunes to subalpine sites [49,83,285]. They occur primarily in wetlands, floodplains, bottomlands, and riparian communities throughout their ranges, but they also occur frequently in upland hardwood, mixed hardwood-conifer, and conifer forests and woodlands (e.g., [49,71,83,174,233,250]). In forests, they often occur in canopy gaps and on edges (see Shade tolerance). They also occur in prairies and other grasslands (e.g., [3,21,111,113,130,134,172,204,240]) as well as on rocky fields, talus slopes, and cliffs [83,171]. See the Fire Regime Table for a list of plant communities in which poison-ivies may occur and information on the FIRE REGIMES associated with those communities.
In the West, western poison-ivy most often occurs, but is seldom predominant, in aspen and cottonwood (Populus spp.), ash, maple, and birch (Betula spp.) gallery forests and floodplain communities (e.g., [27,86,102,105,178,198]). Among shrublands, it is most common in western snowberry (Symphoricarpos occidentalis) [44,104,259], red-osier dogwood (Cornus sericea) [102,198], chokecherry (Prunus virginiana) [103,105,105,198], silver buffaloberry (Shepherdia argentea) [103,105], and willow (Salix spp.) [102,103,105] communities. Along the Little Missouri River in North Dakota, western poison-ivy was abundant in green ash/western snowberry communities [104], and it was common in eastern cottonwood/Rocky Mountain juniper (Populus deltoides/Juniperus scopulorum) communities [85]. In southern Idaho, western poison-ivy occurred with the greatest mean cover in quaking aspen (P. tremuloides)/red-osier dogwood communities, with less cover in red-osier dogwood, Utah juniper (J. osteosperma)/red-osier dogwood, black cottonwood (P. balsamifera subsp. trichocarpa), and willow communities [102]. In Montana, western poison-ivy had the greatest cover in eastern cottonwood/western snowberry, boxelder/chokecherry, and Rocky Mountain juniper/red-osier dogwood communities. It also occurred in a silver buffaloberry community where animal trampling had created gaps in the canopy [103,105]. In Alberta, western poison-ivy was the 4th most abundant shrub in eastern cottonwood/western snowberry communities on alluvial river bars, and it occurred in silver buffaloberry communities on alluvial floodplain terraces [259].
Poison-ivies are common and occasionally important or predominant in upland forests, particularly those with oak, hickory, maple, eastern redcedar (Juniperus virginiana), and pines (e.g., Virginia pine (Pinus virginiana), loblolly pine (P. taeda), shortleaf pine (P. echinata), and eastern white pine (P. strobus)) (e.g., [54,174,177,190,228,254,270]). They were among the most abundant species in Virginia pine forests in Virginia [190,254] and eastern redcedar woodlands in Pennsylvania, New Jersey, and Virginia [190,195]. Poison-ivies are especially common in upland oak forests and woodlands [138,174,190]. Bur oak (Q. macrocarpa), northern pin oak, black oak (Q. velutina), white oak, and post oak (Q. stellata) are typical overstory dominants [54,110,117,138,149,174,194]. In Indiana, eastern poison-ivy was a dominant species in a black oak/Carolina rose (Rosa carolina)-eastern poison-ivy savanna on an open valley site and in a black oak/eastern poison-ivy/woodland sunflower (Helianthus divaricatus) savanna on a south-facing slope [149]. In central Minnesota, western poison-ivy was dominant in a northern pin oak-bur oak/bluegrass (Poa spp.) community and a northern pin oak-bur oak/big bluestem savanna [54]. In Wisconsin, poison-ivies were understory dominants in a white oak-northern pin oak/leadplant community and were of secondary importance in a northern pin oak/wintergreen-New Jersey tea (Gaultheria procumbens-Ceanothus americanus) community and an eastern white pine/hog peanut (Amphicarpa bracteata) community [138]. Western poison-ivy dominated a bur oak-western poison-ivy grove in Manitoba [194].
In the West, western poison-ivy occasionally occurs in uplands, but it is rarely important or predominant (e.g., [70,85,103,117,148]). On upper slopes in North Dakota, it was abundant in quaking aspen/water birch communities [104], and it was less abundant in quaking aspen/chokecherry and quaking aspen/paper birch (Betula papyrifera) communities on upper slopes [85]. Western poison-ivy is a minor species in upland ponderosa pine (Pinus ponderosa) forests in Nevada [198], Montana [103,105], and Wyoming [117] and in Rocky Mountain juniper and Utah juniper woodlands in Idaho [102], Montana [103,105], and North Dakota [85]. In Fort Bayard, New Mexico, western poison-ivy was a dominant understory shrub in a Gambel oak (Q. gambelii)/western poison-ivy/bottlebrush squirreltail-mutton grass-pinyon ricegrass (Elymus elymoides subsp. hordeoides-Poa fendleriana-Piptochaetium fimbriatum) community type [162].
Shoreland communities: Along the Gulf and Atlantic coasts, poison-ivies are common in maritime hammocks and on sand dunes along shores and barrier islands [16,16,49,60,62,71,136,285]. When western poison-ivy occurs on sand dunes, it is usually missing from adjacent dune forests [83], whereas eastern poison-ivy is frequently reported in these forests [94,151]. Eastern poison-ivy was one of the most abundant species in the American holly (Ilex opaca) forest alliance on lee sides of sand dunes along the Atlantic coast from New Jersey to Massachusetts [136]. On sand dunes from Delaware north to central Maine, it was one of the most abundant species in the northern bayberry-beach plum (Prunus maritima) shrubland alliance on protected sand dunes. In coastal areas from southern New Hampshire to New Jersey, it was one of the most abundant species in the chokecherry-Canadian serviceberry-oak (Prunus serotina-Amelanchier canadensis-Quercus spp.) shrubland alliance on sheltered back dunes, bluffs, and interior coastal areas. It was a dominant species in the cat greenbrier (Smilax glauca)-eastern poison-ivy vine-shrubland alliance on sand dunes in New England south to Maryland [136,257].
Wetlands: Eastern poison-ivy is common and often important or predominant in a variety of wetlands including forested swamps, scrub-shrub wetlands, and tidal and nontidal marshes [71,137]. It was the 3rd most common understory plant in pondcypress heads in north-central Florida [169], and it was a common understory species in baldcypress swamps in Florida [62,169], Georgia [219], North Carolina [175,216], and Illinois [58]. On barrier islands along the North Carolina coast, eastern poison-ivy dominated red maple-swamp tupelo (Nyssa sylvatica var. biflora)/eastern poison-ivy maritime swamp forest [216]. In New York, it was a characteristic species in silver maple-ash swamps and was of secondary importance in freshwater tidal swamps dominated by ash, red maple, slippery elm (U. rubra), and American hornbeam (Carpinus caroliniana) [210]. Poison-ivies were among the most abundant species in a silky dogwood (Cornus amomum) palustrine shrubland in the Delaware Water Gap [195]. Eastern poison-ivy dominated a wax-myrtle (Myrica cerifera)-eastern poison-ivy/sand cordgrass (Spartina bakeri) tidal shrub swamp in Virginia [191], and it was common in the common reed tidal salt marsh herbaceous alliance along the Atlantic coast [136]. It was an important species in a common cattail (Typha latifolia) community in North Carolina [7]. Western poison-ivy was codominant with common reed (Phragmites australis) in riparian communities in Idaho [128].
Grasslands: Poison-ivies are common, but rarely important or predominant, in many grasslands [3,21,107,111,114,130,201]. They occurred in indiangrass (Sorghastrum nutans) tallgrass prairie in Oklahoma [3], in big bluestem-indiangrass-little bluestem (Schizachyrium scoparium var. scoparium) tallgrass prairie in northeastern Nebraska [111], and in big bluestem-prairie dropseed (Andropogon gerardii-Sporobolus heterolepis) prairie in southwestern Minnesota [21]. In North Dakota, western poison-ivy occurred in a little bluestem-creeping juniper (Juniperus horizontalis) community on rocky soils, a shrubby cinquefoil (Dasiphora fruticosa spp. floribunda)-little bluestem community on steep, upland slopes, and a big bluestem community in moist depressions [114]. It was a common species on prairie sandreed-needle-and-thread grass (Calamovilfa longifolia-Hesperostipa comata) sand dunes of the Niobrara Valley Preserve, Nebraska [107]. In southeastern Montana, western poison-ivy occurred on uplands in xeric western wheatgrass/blue grama (Pascopyrum smithii/Bouteloua gracilis) grasslands [130].
Rocky outcrops, talus slopes, and cliffs: Poison-ivies are common and often important or predominant on rocky outcrops, talus slopes, and cliffs (e.g., [116,174,176,195,247,258]). They were dominant in the poison-ivy-granite gooseberry-pawpaw (Ribes curvatum-Asimina triloba) shrub-vineland association on scree fields and talus slopes in the Ouachita Mountains, Oklahoma [116]. Eastern poison-ivy dominated the sparsely covered eastern poison-ivy/Cossatot Mountain leafcup (Polymnia cossatotensis) vegetation type on steep, unstable talus slopes on Tom, Blaylock, and Peter mountains, Arkansas [174]. On dry cliffs of Pisgah National Forest, North Carolina, eastern poison-ivy dominated the eastern poison-ivy/American alumroot (Heuchera americana) herbaceous vegetation association [176]. In Acadia National Park, Maine, western poison-ivy was a characteristic species of the rock polypody-Appalachian polypody (Polypodium virginianum-P. appalachianum) vegetation community on open talus slopes [154].Germination tests from soils suggest that poison-ivies may form a persistent seed bank [13,24,25,46,67,157]. Because seeds retained on the plant over winter are dormant, poison-ivies also have a temporary aboveground seed reserve [285].
Many researchers have reported poison-ivies in seed banks. In a maple bottomland swamp in western New York, where mean eastern poison-ivy cover was 3%, seedling emergence tests in a greenhouse indicated a mean density of 0.1 seed/120 cm² in the upper 2 inches (5 cm) of soil in April [25]. Using soil samples collected from eastern white pine, red pine (Pinus rubra), and Virginia pine plantations in southern Ohio, seedling emergence tests in a greenhouse indicated a mean density of 117 poison-ivy seeds/m² in the upper 4 inches (10 cm) of mineral soil [13]. In an old field (11 years since cultivation) in Prince George's County, Maryland, seedling emergence tests in a greenhouse indicated a mean volume of 11 poison-ivy seeds/1,000 cm³ in the upper 3 inches (8 cm) of soil in late November and early December [24]. In an upland oak-pine forest in New Jersey, seedling emergence tests in a greenhouse indicated a mean density of 10 poison-ivy seeds/m² in the upper 4 inches (10 cm) of soil collected in June and July [157]. In eastern Tennessee, 1,626 eastern poison-ivy seedlings/ha germinated from seeds in topsoil the first growing season after the topsoil was removed from mixed-hardwood stands and spread over surface mine soils [67].
In contrast, other researchers reported that seeds of poison-ivies either failed to germinate from or were sparse in soil samples despite abundance in the standing vegetation (e.g., [41,56,211]). Leicht-Young and others [149], observing that eastern poison-ivy was dominant in the aboveground vegetation in a black oak savanna but did not germinate from soil samples in a greenhouse, concluded that eastern poison-ivy does not commonly form a seed bank. Eastern poison-ivy was the most common understory plant in a midsuccessional sweetgum-loblolly pine-red maple forest in Virginia, with an average of 41% cover. However, seedling emergence tests in a greenhouse indicated a mean density of only 1 seedling/m² in the upper 3 inches (8 cm) of soil in November [177]. Although western poison-ivy relative abundance was 16% in the Garden Canyon floodplain in the Huachuca Mountains of Arizona, no western poison-ivy seedlings germinated in the laboratory from samples taken from the upper 2 inches (5 cm) of soil in March [211]. In a loblolly pine plantation in North Carolina, eastern poison-ivy was present in the aboveground vegetation, but no seedlings emerged during greenhouse germination tests from samples taken from the upper 4 inches (10 cm) of soil in August and May [41].
Because male and female flowers are on separate plants, not all poison-ivy plants bear fruit [215]. In sand dunes along Lake Erie, 47% of plants bore seeds, with an average of 131 seeds/shoot [285].
Poison-ivy fruit and seed production is often high. An eastern poison-ivy plant with a 3.5-inch (9 cm) diameter main stem had a 4.3-foot (1.3 m) long branch with 6,130 flowers [84]. In September in Maryland, before birds began harvesting seeds, poison-ivies had an average of 315 fruits/plant [52]. In sand dunes along Lake Erie, they had 2,165 fruits/m² in April [285]. In Manitoba, western poison-ivy plants averaged 26 fruits/plant in January and early February; 49 days later there was only an average of 1 fruit/plant because most were consumed by birds and squirrels [194].
Some researchers reported low seed production in poison-ivies. In hardwood forest on the Pisgah National Forest, North Carolina, poison-ivies never fruited in 5 years [93]. Although poison-ivies were abundant in both logged and unlogged shortleaf pine-mixed hardwood stands in west-central Arkansas and east-central Oklahoma, they produced few fruits [196].
Poison-ivies first produce seed at 3 years old [81].
Soils: Poison-ivies grow best in fertile, moist but well-drained soils, although they tolerate a wide range of fertility, moisture, and other conditions. They occur on soils ranging from xeric, shallow rocky soils with southern exposure, to mesic soils on northerly and sheltered exposures, to saturated soils in seeps [216]. Poison-ivies grow in talus and in crevices on steep cliffs as well as in deep soils (>3 feet (1 m)) [174].
Texture: Poison-ivies grows in soils of all textures, including clays, silts, loams, and sands (e.g., [10,33,44,75,80,85,98,130,234,240]). According to Mulligan and Junkins [171], western poison-ivy generally occurs on sandier soils than eastern poison-ivy. Poison-ivies also occur in areas dominated by boulders, stones, cobbles, and gravels [70,130,148,198,223,234,240,268], including talus slopes, cliffs, and rocky ridges [174,234].
pH and parent materials: Poison-ivies occur in extremely acidic to moderately alkaline soils. Eastern poison-ivy occurred in soils with pH ranging from 3.6 to 6.5 [13,80,83]. It increased as soil pH increased in pondcypress (Taxodium distichum var. imbricarium) domes in north-central Florida [169]. Western poison-ivy generally occurs in more alkaline soils than eastern poison-ivy [84,171]. It occurred in soils with pH ranging from 5.7 to 8.4 [75,85,130]. Poison-ivies occur in soils derived from most parent materials (e.g., [75,83,91,130,140,160,174,214,216]).
Nutrients: Poison-ivies occur in "rich" soils [84,133,240] as well as in nutrient-poor soils (e.g., [12,33,98]). They were dominant in the understory of a white oak-northern pin oak/leadplant (Quercus ellipsoidalis/Amorpha canescens) community in Wisconsin that had dry, fine sands with poor to medium nutrient content [138].
Poison-ivies appear to prefer soils with high calcium content. Eastern poison-ivy appears tolerant of high phosphorus levels. In the laboratory, seeds of poison-ivies in a calcium-poor solution germinated but seedlings died soon after [84]. Eastern poison-ivy increased with increased soil calcium in pondcypress heads in north-central Florida [169]. In Florida, eastern poison-ivy was an important component of 100-year-old baldcypress (Taxodium distichum var. distichum) stands with high phosphorus levels [179].
Moisture: Poison-ivies occur on very dry to very wet sites and on poorly drained to well-drained soils (e.g., [33,80,110,112,157,174,216,248]), but generally they prefer well-drained, mesic soils (e.g., [2,47,70,123,135,158,214,234,250,260]). For example, in mixed-mesophytic forest in Indiana, eastern poison-ivy occurred on some xeric sites but had highest cover on mesic sites [123]. In mixed-oak (Quercus spp.) forest in southern Ohio, poison-ivies occurred with lower frequency on xeric sites (10.3%) than on intermediate (16.9%) or mesic (15.1%) sites [127].
Throughout their ranges, poison-ivies commonly occur in wetlands [23,170,208]. They often occur in seasonally or intermittently flooded areas (e.g., [58,89,118,216]) as well as areas such as marshes and swamps that are flooded for long periods (e.g., [198,216]). On these sites, poison-ivies often occur on elevated microsites such as on hummocks or tree bases [73,174]. Eastern poison-ivy on north-central Florida pondcypress heads decreased in importance with increased flooding depth; most eastern poison-ivy plants were found on large, elevated peat mats around the bases of pondcypress trees [169]. In Piatt County, Illinois, poison-ivies were the most frequently occurring woody species on flood-prone sites in silver maple (Acer saccharinum) streamside forests; these sites were inundated more than 20% of the time during the historical record. However, poison-ivies also occurred in upland white oak (Quercus alba) forests that were never flooded [22]. On wet meadows and other riparian prairie communities along the Middle Loup and Loup rivers in central Nebraska, poison-ivies increased with increased groundwater depth [172].
Poison-ivies appear tolerant of flooding [73]. Observations at the Montezuma National Wildlife Refuge, New York, suggested that eastern poison-ivy density declined 1 year after controlled flooding of 2 red maple-green ash (Acer rubrum-Fraxinus pennsylvanica) wetland reservoirs. Two years after flooding, eastern poison-ivy density had increased but remained below that on control sites. Eighteen years after flooding, density on flooded sites exceeded that on the controls [53]. A bottomland hardwood forest along the Mississippi River in Louisiana was sampled before and after a 105-day flood. Submerged eastern poison-ivy plants were top-killed by the flooding, while those with leaves above water remained green. The lower portion of the stem sprouted "vigorously" after the flood water receded. Forty-three days after the flood, eastern poison-ivy cover was greater than that before the flood, although the difference was not statistically significant [183].
Poison-ivies appear intolerant of drought. Western poison-ivy in the prairies of eastern Nebraska, western Iowa, and Kansas was injured or killed by the severe drought of the 1930s [271]. In Tennessee, the number of eastern poison-ivy growing points was reduced by half during a severe drought in the 1980s [68]. In New Jersey, eastern poison-ivy cover varied in response to drought among 6 old fields. In one field, it was reduced by 58%, but in another field it increased 3%. Overall, eastern poison-ivy cover in the old fields declined during the drought. It returned to predrought levels in 2 years. The authors considered eastern poison-ivy a "drought-susceptible" species [284].
Salinity: Poison-ivies are tolerant of mildly saline water [60,62,73,136] and soils [130] and can tolerate light to moderate salt spray [94,173,216].
Poison-ivies tolerate both sun and shade. They are generally rated as moderately shade tolerant [140,143]. They are common from the first stages of plant succession to late succession [97], being most common in early to midsuccession (see Successional stage). Poison-ivies commonly occur on disturbed sites such as floodplains, sand dunes, and talus slopes (e.g., [60,83,95,156,156,204,268,283]) and often increase after disturbances that open the canopy, such as fire, windstorms, forest pathogen outbreaks, and logging. Because of poison-ivies' affinity for forest edges and canopy gaps, researchers suggested poison-ivies are likely to increase with forest fragmentation [34,120].
Shade tolerance: Poison-ivies occur in sunny to shaded sites. They are most abundant in moderately shady sites (e.g., [8,29,47,83,87,109,238]). Gillis [83] stated that western poison-ivy is seldom found in closed-canopy forests because it is likely to be shaded out. Eastern poison-ivy may be better able persist in closed-canopy forests than western poison-ivy because of its ability to climb into the forest canopy and access light [143]. Ladwig and Meiners [143] suggested that eastern poison-ivy employs a "sit-and-wait" strategy in late succession by persisting at low cover in the forest canopy until treefall gaps and other canopy-opening disturbances allow it to spread. In Minnesota, frequency of poison-ivies in northern pin oak-bur oak woodlands was about 15% when the canopy was 8% closed. Their frequency peaked at 55% when the canopy was 22% closed. As canopy closure increased, frequency of poison-ivies declined steadily. When the canopy was 75% closed, their frequency was as low as their frequency in the most open sites (15%). They persisted at very low frequency (2%) even when the canopy was 100% closed [197]. In maple-beech (Fagus spp.) "climax" forest in northern Minnesota, western poison-ivy was "suppressed and scattered" in dense shade but more abundant in areas with more light [238]. In southeastern Ohio, eastern poison-ivy frequency was greater in 2nd-growth oak forest than in old-growth forest, apparently due to relatively greater light availability in 2nd-growth oak forest [184]. Poison-ivies showed an affinity for open microsites in a 73-year-old northern mixed-mesophytic hardwood forest in Ithaca, New York [78]. Eastern poison-ivy decreased as canopy closure increased during 15 years in an old-growth oak-hickory forest in southwestern Illinois. The authors concluded that eastern poison-ivy was "intolerant of heavy shade" [225]. In contrast, its importance value increased 2.3-fold in a mixed pine-hardwood baygall in west-central Louisiana during 15 years. Because its abundance increased as the canopy closed, eastern poison-ivy was described as shade tolerant [6].
Abundance of poison-ivies is often higher on forest edges than in forest interiors (e.g., [34,74,120,152]). Londre and Schnitzer [152] hypothesized that the high abundance of eastern poison-ivy at forest edges may be due to increased temperature, decreased relative humidity, increased wind turbulence, and trellis availability near forest edges. Eastern poison-ivy may also colonize forest edges rapidly because of seed dispersal by avian frugivores, which generally spend more time consuming fruits and depositing seeds along edges than within interiors of temperate forests [152]. Hardin [106] observed that encroachment of poison-ivies into a southeastern Ohio prairie occurred mostly under overhanging tree limbs. Poison-ivies were among the most abundant species on oak forest edges on the Shawnee National Forest, Illinois. They were abundant up to 164 feet (50 m) from the forest edge into the forest interior but declined at greater distances [120]. In the Roanoke River basin, North Carolina, eastern poison-ivy occurred within 66 feet (20 m) of the edge of mixed-hardwood forests. It penetrated deeper into the forest on south-facing edges than north-facing edges [74]. In a hardwood forest in Massachusetts, trees near the edge of the forest supported more eastern poison-ivy stems than those in the forest interior, presumably because of greater light availability [34]. In Wisconsin, eastern poison-ivy density was 2 times greater at the forest edge than in the forest interior (P<0.05). Abundance decreased sharply at 16 feet (5 m) from the edge. Edge sites had the highest light availability and the lowest canopy density (P≤0.05 for both variables) [152]. A 50-year study examined eastern poison-ivy population expansion into an abandoned field in the Hutcheson Memorial Forest, New Jersey. Population expansion in years 10 to 25 was greatest near the edge of the bordering old-growth forest and decreased with increasing distance into the field. By years 30 to 40, the population near the edge of the old-growth forest was declining [143].
Poison-ivies often establish in forest gaps (e.g., [9,90,239]). In Madison County, New York, they were described as "gap species" in northern whitecedar-balsam fir (Thuja occidentalis-Abies balsamea) forest because they had a higher importance value in medium (650-910 feet ² (60-85 m²)) and large (1,290-2,050 feet² (120-190 m²)) canopy gaps than in closed-canopy forest (P<0.05) [9]. Eastern poison-ivy was an important species in old-growth shortleaf pine forest in Missouri, where canopy gaps (≤6 years old) constituted 4% of the total area and gap size averaged 2,260 feet² (210 m²) [239]. In contrast, in New London, Connecticut, frequency of poison-ivies in an eastern hemlock (Tsuga canadensis)-mixed hardwood forest did not change substantially (10%-17%) over 45 years despite tree mortality and opening of the forest overstory due to hemlock woolly adelgid [90].
Logging creates openings in the forest canopy that often benefit poison-ivies. On the Nantahala National Forest, North Carolina, poison-ivies were present in a northern red oak/flame azalea (Rhododendron calendulaceum) forest after hurricane windthrow and posthurricane logging had created 0.25- to 0.50-acre (0.1-0.2 ha) openings in the canopy; however, they were absent from an adjacent unlogged forest [64]. In the Tennessee National Wildlife Refuge, thinned oak-hickory stands had greater mean eastern poison-ivy cover than unthinned stands 4 years after logging [256]. Mean eastern poison-ivy density was higher in southern pine beetle-infested and logged loblolly pine stands (290,080 stems/ha) than in undisturbed stands (16,950 stems/ha) in Turkey Hill Wilderness, Texas (P<0.05) [171]. In contrast, mean eastern poison-ivy density was similar between southern pine beetle-infested and logged loblolly pine stands (24,720 stems/ha) and undisturbed stands (123,775 stems/ha) in Indian Mounds Wilderness, Texas [43].
Poison-ivies often increase substantially after windstorms that open the forest canopy. On Long Pine Key, Florida, eastern poison-ivy and other lianas and vines grew rapidly in maritime hammocks within 1 month after Hurricane Andrew, apparently in response to the canopy opening [153]. Eastern poison-ivy was "prolific" the first growing season after Hurricane Hugo removed much of the overstory of an old-growth maritime forest on Bull Island, South Carolina [231]. In a mixed-hardwood forest in Ottawa, Ontario, eastern poison-ivy seedlings were "more abundant" during the 4 years following an ice storm than before the storm [51]. Near Franklin, North Carolina, frequency, density, and cover of poison-ivies increased from 1 to 3 years after salvage logging of a high-elevation hickory-black oak-yellow-poplar (Liriodendron tulipifera) stand damaged by Hurricane Opal. Poison-ivies were not present in undisturbed stands [64].
The effect of windstorms on abundance of poison-ivies is influenced by the amount of canopy removed, although the effect is unclear. A study in Minnesota found that poison-ivies increased most in the most severely hurricane-damaged areas [189], whereas a study in South Carolina reported that eastern poison-ivy increased most in less severely damaged areas [5]. In Cedar Creek National Historical Park, Minnesota, eastern poison-ivy frequency in an eastern white pine forest increased 19% fourteen years after a windstorm caused high tree mortality, but in a northern pin oak forest, eastern poison-ivy frequency increased 14% during postdisturbance years 1 to 7, then declined to predisturbance levels. The authors suggested that eastern poison-ivy increased more in the eastern white pine forest because the storm damaged that forest more than it damaged the northern pin oak forest [189]. In an old-growth bottomland hardwood forest in Congaree National Park, South Carolina, eastern poison-ivy response to damage from Hurricane Hugo varied with damage severity. In the most severely damaged areas, density decreased 55% in the 1st year after the hurricane and remained low through year 5. Eight years after the hurricane, however, eastern poison-ivy density had nearly doubled, and by posthurricane year 12, it exceeded that before the hurricane. In areas with less severe damage, eastern poison-ivy density decreased initially, but only slightly. By year 5, density exceeded that before the hurricane, and it continued to increase through the end of the study in year 12 [5].
Successional stage: Poison-ivies occur in every stage of succession. However, they often increase after disturbance, and they are typically most common in early to midsuccession. They often persist into later stages of succession, but in lesser abundance [83,86]. In late-successional forests, they often persist in canopy gaps or along forest edges (see Shade tolerance). Because of its ability to access light in the forest canopy, eastern poison-ivy appears more common in late succession than western poison-ivy.
Although sometimes common in early succession, poison-ivies typically reach peak abundance during midsuccession. Studies of old-field succession reported that poison-ivies were often absent immediately after agricultural abandonment but increased soon after. For example, eastern poison-ivy was one of the earliest woody species to invade old fields in southeastern Ontario; it was observed within 3 years of abandonment [48]. Cover of poison-ivies typically peaks in midsuccession, from about 20 years to 60 years after abandonment, then declines in late succession, when poison-ivies often persist in forest gaps or along forest edges (e.g., [18,48,69,100,121,143,200,203,264]).
Table 2. Observations of poison-ivy abundance in old fields after agricultural abandonment Species Location Observations eastern poison-ivy southeastern Connecticut present in trace amounts 9 years after abandonment 28 years after abandonment, when the old field was a thicket of trees and shrubs 13-20 feet (4-6 m) tall, cover was 3% cover remained similar (2%-4%) 38 and 47 years after abandonment [69] New Jersey present 1 year after abandonment 4% cover 15 years after abandonment, when old fields were open and "park-like" and eastern redcedars were 7-8 feet (2-2.5 m) tall 8% cover 40 years after abandonment 30% cover 60 years after abandonment, when old fields were eastern redcedar groves with a thicket understory [18] present within 7 years of abandonment cover peaked 22 years after abandonment (15%), when young trees began to provide support cover decreased to about 5% by year 50 [143] southwestern Ohio absent 2 and 10 years after abandonment common 50 years after abandonment present only in disturbed areas 90 and >200 years after abandonment [264] central Tennessee absent from herb-dominated old fields, 1-12 years after abandonment present in shrub-herb thickets and young forests, 8-20 years after abandonment peak cover in elm and hackberry (Celtis spp.) forests, the oldest fields studied, 25 years after abandonment [203] poison-ivies central New Jersey absent from old fields in years 1-6 after abandonment present in low abundance 7 years after abandonment cover increased steadily until 20 years after abandonment when the study ended [200] southeastern Indiana absent 1 and 2 years after abandonment present 3 and 10 years after abandonment [121]Poison-ivies are common in infrequently to frequently disturbed floodplains and lakeshores [83]. On floodplain sites along the Little Missouri River in North Dakota, highest western poison-ivy cover (18%) occurred on the most recent alluvial deposits in eastern cottonwood communities. Its cover gradually declined as the overstory canopy closed. In a "climax" green ash/western snowberry community, its cover was only 4% [86]. In contrast, in South Dakota, eastern poison-ivy occurred in 35-year-old and 50-year-old eastern cottonwood stands in floodplains along the Missouri River but not in 10-, 14-, or 23-year-old stands [280]. Along the Yellowstone River in Wyoming, western poison-ivy was absent from seedling and sapling eastern cottonwood stands and had <1% cover in pole eastern cottonwood stands, but it had 12% cover in mature eastern cottonwood stands [27].
Poison-ivies often occur in postfire successional communities. For more information on this topic, see Plant response to fire.The genus name for poison-ivy is Toxicodendron Mill. (Anacardiaceae). This review summarizes information on the following poison-ivy taxa:
Toxicodendron radicans (L.) Kuntze, eastern poison-ivy [40,87,88,92,124,137,168,266,281,283].
Toxicodendron rydbergii (Small ex. Rydb.) Greene, western poison-ivy [29,38,47,83,132,156,240,263].
Nine subspecies of eastern poison ivy are recognized globally, 6 of which occur in the United States:
Toxicodendron radicans subsp. radicans (L.) Kuntze [55,88,132,156,247,283]
Toxicodendron radicans subsp. divaricatum (Greene) Gillis [83,263]
Toxicodendron radicans subsp. eximium (Greene) Gillis [55,132,202,263]
Toxicodendron radicans subsp. negundo (Greene) Gillis [55,92,132,240,263,268,283]
Toxicodendron radicans subsp. pubens (Engelm. ex Watson) [55,263,283]
Toxicodendron radicans subsp. verrucosum (Scheele) Gillis [55,83,92,132,263]
Eastern and western poison-ivy are morphologically plastic [81] and intergrade with one another [83,87,171]. They occasionally hybridize where their ranges overlap [47,83,92,268]. Eastern poison-ivy may hybridize with Atlantic poison-oak (T. pubescens), and western poison-ivy may hybridize with Pacific poison-oak (T. diversilobum) [83].
Older literature often does not distinguish between eastern and western poison-ivy, and because the 2 species' geographic distributions overlap, it is sometimes impossible to determine to which species older literature is referring. In this review, species are referred to by their common names, when possible, and "poison-ivies" refers to both species.
Toxicodendron radicans, commonly known as eastern poison ivy[1] or poison ivy, is an allergenic Asian and Eastern North American flowering plant in the genus Toxicodendron. The species is well known for causing urushiol-induced contact dermatitis, an itchy, irritating, and sometimes painful rash, in most people who touch it. The rash is caused by urushiol, a clear liquid compound in the plant's sap.[2] The species is variable in its appearance and habit, and despite its common name, it is not a true ivy (Hedera), but rather a member of the cashew and pistachio family (Anacardiaceae). T. radicans is commonly eaten by many animals and the seeds are consumed by birds,[3] but poison ivy is most often thought of as an unwelcome weed. It is a different species from western poison ivy, T. rydbergii, which has similar effects.
Numerous subspecies and/or varieties of T. radicans are known.[4] They can be found growing in any of the following forms, all having woody stems:
The deciduous leaves of T. radicans are trifoliate with three almond-shaped leaflets.[5] Leaf color ranges from light green (usually the younger leaves) to dark green (mature leaves), turning bright red in fall; though other sources say leaves are reddish when expanding, turn green through maturity, then back to red, orange, or yellow in the fall. The leaflets of mature leaves are somewhat shiny. The leaflets are 3–12 cm (1+1⁄4–4+3⁄4 in) long, rarely up to 30 cm (12 in). Each leaflet has a few or no teeth along its edge, and the leaf surface is smooth. Leaflet clusters are alternate on the vine, and the plant has no thorns. Vines growing on the trunk of a tree become firmly attached through numerous aerial rootlets.[6] The vines develop adventitious roots, or the plant can spread from rhizomes or root crowns. The milky sap of poison ivy darkens after exposure to the air.
T. radicans spreads either vegetatively or sexually. It is dioecious; flowering occurs from May to July. The yellowish- or greenish-white flowers are typically inconspicuous and are located in clusters up to 8 cm (3 in) above the leaves. The berry-like fruit, a drupe, mature by August to November with a grayish-white colour.[5]
T. radicans in Perrot State Park, Trempealeau County, Wisconsin
These four characteristics are sufficient to identify poison ivy in most situations: (a) clusters of three leaflets, (b) alternate leaf arrangement, (c) lack of thorns, and (d) each group of three leaflets grows on its own stem, which connects to the main vine, the middle stem is longer.[7] The appearance of poison ivy can vary greatly among environments, and even within a large area. Identification by experienced people is often made difficult by leaf damage, the plant's leafless condition during winter, and unusual growth forms due to environmental or genetic factors. Various mnemonic rhymes describe the characteristic appearance of poison ivy:[8]
Caquistle or caxuistle is the Nahuatl term for the species.
T. radicans grows throughout much of North America, including the Canadian Maritime provinces, Quebec, Ontario, and all US states east of the Rocky Mountains,[15] as well as in the mountainous areas of Mexico up to around 1,500 m (4,900 ft). It is normally found in wooded areas, especially along edge areas where the tree line breaks and allows sunshine to filter through. It also grows in exposed rocky areas, open fields, and disturbed areas.
It may grow as a forest understory plant, although it is only somewhat shade-tolerant.[5] The plant is extremely common in suburban and exurban areas of New England, the Mid-Atlantic, and the Southeastern United States. The similar species T. diversilobum (western poison oak) and T. rydbergii (western poison ivy) are found in western North America, and T. orientale in Taiwan, Japan, Korea and Sakhalin.
T. radicans rarely grows at altitudes above 1,500 m (4,900 ft), although the altitude limit varies in different locations.[5] The plants can grow as a shrub up to about 1.2 m (4 ft) tall, as a groundcover 10–25 cm (4–10 in) high, or as a climbing vine on various supports. Older vines on substantial supports send out lateral branches that may be mistaken for tree limbs at first glance.
It grows in a wide variety of soil types, and soil pH from 6.0 (acidic) to 7.9 (moderately alkaline). It is not particularly sensitive to soil moisture, although it does not grow in desert or arid conditions. It can grow in areas subject to seasonal flooding or brackish water.[5]
It is more common now than when Europeans first arrived in North America. The development of real estate adjacent to wild, undeveloped land has engendered "edge effects", enabling poison ivy to form vast, lush colonies in these areas. It is listed as a noxious weed in the US states of Minnesota and Michigan and in the Canadian province of Ontario.
Outside North America, T. radicans is also found in parts of China.[14]
Poison ivy is particularly sensitive to carbon dioxide levels, greatly benefiting from higher concentrations in the atmosphere. Higher carbon dioxide levels increase the rate of plant growth, and cause them to produce more unsaturated urushiol, which causes stronger reactions in humans.[16] Poison ivy's growth and potency has already doubled since the 1960s, and it could double again once carbon dioxide levels reach 560 ppm.[17]
The fruits are a favorite winter food of some birds and other animals, including wild turkeys.[18] Seeds are spread mainly by animals and remain viable after passing through the digestive tract. Birds may spread the seeds by regurgitation.[19]
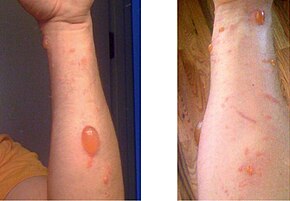
Urushiol-induced contact dermatitis is the allergic reaction caused by poison ivy. In extreme cases, a reaction can progress to anaphylaxis. Around 15 to 25 percent of people have no allergic reaction to urushiol, but most people have a greater reaction with repeated or more concentrated exposure.[20][21] Typically, the rash from the urushiol oil lasts about five to twelve days, but in extreme cases, it can last a month or more.[22]
Over 350,000 people are affected by urushiol annually in the United States.[23]
The pentadecyl catechols of the oleoresin within the sap of poison ivy and related plants causes the allergic reaction; the plants produce a mixture of pentadecylcatechols, which collectively is called urushiol. After injury, the sap leaks to the surface of the plant where the urushiol becomes a blackish lacquer after contact with oxygen.[2][24]
Urushiol binds to the skin on contact, where it causes severe itching that develops into reddish inflammation or uncoloured bumps, and then blistering. These lesions may be treated with Calamine lotion, Burow's solution compresses, dedicated commercial poison ivy itch creams, or baths to relieve discomfort,[25] though recent studies have shown some traditional medicines to be ineffective.[26][27] Over-the-counter products to ease itching—or simply oatmeal baths and baking soda—are now recommended by dermatologists for the treatment of poison ivy.[28]
A plant-based remedy cited to counter urushiol-induced contact dermatitis is jewelweed, though jewelweed extracts had no positive effect in clinical studies.[29][30][31][32] Others argue that prevention of lesions is easy if one practices effective washing, using plain soap, scrubbing with a washcloth, and rinsing three times within 2–8 hours of exposure.[33]
The oozing fluids released by scratching blisters do not spread the poison. The fluid in the blisters is produced by the body and it is not urushiol itself.[34] The appearance of a spreading rash indicates that some areas received more of the poison and reacted sooner than other areas or that contamination is still occurring from contact with objects to which the original poison was spread. Those affected can unknowingly spread the urushiol inside the house, on phones, doorknobs, couches, counters, desks, and so on, thus in fact repeatedly coming into contact with poison ivy and extending the length of time of the rash. If this has happened, wipe down the surfaces with bleach or a commercial urushiol removal agent. The blisters and oozing result from blood vessels that develop gaps and leak fluid through the skin; if the skin is cooled, the vessels constrict and leak less.[35] If plant material with urushiol is burned and the smoke then inhaled, this rash will appear on the lining of the lungs, causing extreme pain and possibly fatal respiratory difficulty.[34] If poison ivy is eaten, the mucus lining of the mouth and digestive tract can be damaged.[36] An urushiol rash usually develops within a week of exposure and can last 1–4 weeks, depending on severity and treatment. In rare cases, urushiol reactions may require hospitalization.[34]
Urushiol oil can remain active for several years, so handling dead leaves or vines can cause a reaction. In addition, oil transferred from the plant to other objects (such as pet fur) can cause the rash if it comes into contact with the skin.[37][34] Clothing, tools, and other objects that have been exposed to oil should be washed to prevent further reactions.[38]
People who are sensitive to urushiol can also experience a similar rash from mangoes. Mangoes are in the same family (Anacardiaceae) as poison ivy; the sap of the mango tree and skin of mangoes has a chemical compound similar to urushiol.[39] A related allergenic compound is present in the raw shells of cashews.[40] Similar reactions have been reported occasionally from contact with the related Fragrant Sumac (Rhus aromatica) and Japanese lacquer tree. These other plants are also in the family Anacardiaceae.
Immediate washing with soap and cold water or rubbing alcohol may help prevent a reaction.[41] During a reaction, Calamine lotion or diphenhydramine may help mitigate symptoms. Corticosteroids, either applied to the skin or taken by mouth, may be appropriate in extreme cases. An astringent containing aluminum acetate (such as Burow's solution) may also provide relief and soothe the uncomfortable symptoms of the rash.[42]
Toxicodendron radicans, commonly known as eastern poison ivy or poison ivy, is an allergenic Asian and Eastern North American flowering plant in the genus Toxicodendron. The species is well known for causing urushiol-induced contact dermatitis, an itchy, irritating, and sometimes painful rash, in most people who touch it. The rash is caused by urushiol, a clear liquid compound in the plant's sap. The species is variable in its appearance and habit, and despite its common name, it is not a true ivy (Hedera), but rather a member of the cashew and pistachio family (Anacardiaceae). T. radicans is commonly eaten by many animals and the seeds are consumed by birds, but poison ivy is most often thought of as an unwelcome weed. It is a different species from western poison ivy, T. rydbergii, which has similar effects.