en
names in breadcrumbs


Abstract:
Klebsiella pneumoniae (K. pneumoniae) is a common nosocomial pathogen causing respiratory tract (pneumoniae) and blood stream infections. Multidrug-resistant (MDR) isolates of K. pneumoniae infections are difficult to treat in patients in health care settings. Aim of the present study was to determine the impact of Mr. Trivedi’s biofield treatment on four MDR clinical lab isolates (LS) of K. pneumoniae (LS 2, LS 6, LS 7, and LS 14). Samples were divided into two groups i.e. control and biofield treated. Control and treated groups were analyzed for antimicrobial susceptibility pattern, minimum inhibitory concentration (MIC), biochemical study and biotype number using MicroScan Walk-Away® system. The analysis was done on day 10 after biofield treatment as compared with control group. Antimicrobial sensitivity assay showed that there was 46.42% alteration in sensitivity of tested antimicrobials in treated group of MDR K. pneumonia isolates. MIC results showed an alteration in 30% of tested antimicrobials out of thirty after biofield treatment in clinical isolates of K. pneumoniae. An increase in antimicrobial sensitivity and decrease in MIC value was reported (in LS 6) in case of piperacillin/tazobactam and piperacillin. Biochemical study showed a 15.15% change in biochemical reactions as compared to control. A significant change in biotype numbers were reported in all four clinical isolates of MDR K. pneumoniae after biofield treatment as compared to control group. On the basis of changed biotype number after biofield treatment, new organism was identified as Enterobacter aerogenes in LS 2 and LS 14. These results suggest that biofield treatment has a significant effect on altering the antimicrobial sensitivity, MIC values, biochemical reactions and biotype number of multidrug-resistant isolates of K. pneumoniae.
Klebsiella pneumoniae (K. pneumoniae) is a common nosocomial pathogen causing respiratory tract (pneumoniae) and blood stream infections. Multidrug-resistant (MDR) isolates of K. pneumoniae infections are difficult to treat in patients in health care settings. Aim of the present study was to determine the impact of Mr. Trivedi’s biofield treatment on four MDR clinical lab isolates (LS) of K. pneumoniae (LS 2, LS 6, LS 7, and LS 14). Samples were divided into two groups i.e. control and biofield treated. Control and treated groups were analyzed for antimicrobial susceptibility pattern, minimum inhibitory concentration (MIC), biochemical study and biotype number using MicroScan Walk-Away® system. The analysis was done on day 10 after biofield treatment as compared with control group. Antimicrobial sensitivity assay showed that there was 46.42% alteration in sensitivity of tested antimicrobials in treated group of MDR K. pneumonia isolates. MIC results showed an alteration in 30% of tested antimicrobials out of thirty after biofield treatment in clinical isolates of K. pneumoniae. An increase in antimicrobial sensitivity and decrease in MIC value was reported (in LS 6) in case of piperacillin/tazobactam and piperacillin. Biochemical study showed a 15.15% change in biochemical reactions as compared to control. A significant change in biotype numbers were reported in all four clinical isolates of MDR K. pneumoniae after biofield treatment as compared to control group. On the basis of changed biotype number after biofield treatment, new organism was identified as Enterobacter aerogenes in LS 2 and LS 14. These results suggest that biofield treatment has a significant effect on altering the antimicrobial sensitivity, MIC values, biochemical reactions and biotype number of multidrug-resistant isolates of K. pneumoniae.
Klebsiella pneumoniae (K. pneumoniae) is a common nosocomial pathogen causing respiratory tract (pneumoniae) and blood stream infections. Multidrug-resistant (MDR) isolates of K. pneumoniae infections are difficult to treat in patients in health care settings. Aim of the present study was to determine the impact of Mr. Trivedi’s biofield treatment on four MDR clinical lab isolates (LS) of K. pneumoniae (LS 2, LS 6, LS 7, and LS 14). Samples were divided into two groups i.e. control and biofield treated. Control and treated groups were analyzed for antimicrobial susceptibility pattern, minimum inhibitory concentration (MIC), biochemical study and biotype number using MicroScan Walk-Away® system. The analysis was done on day 10 after biofield treatment as compared with control group. Antimicrobial sensitivity assay showed that there was 46.42% alteration in sensitivity of tested antimicrobials in treated group of MDR K. pneumonia isolates. MIC results showed an alteration in 30% of tested antimicrobials out of thirty after biofield treatment in clinical isolates of K. pneumoniae. An increase in antimicrobial sensitivity and decrease in MIC value was reported (in LS 6) in case of piperacillin/tazobactam and piperacillin. Biochemical study showed a 15.15% change in biochemical reactions as compared to control. A significant change in biotype numbers were reported in all four clinical isolates of MDR K. pneumoniae after biofield treatment as compared to control group. On the basis of changed biotype number after biofield treatment, new organism was identified as Enterobacter aerogenes in LS 2 and LS 14. These results suggest that biofield treatment has a significant effect on altering the antimicrobial sensitivity, MIC values, biochemical reactions and biotype number of multidrug-resistant isolates of K. pneumoniae.
Abstract:
Pathogenic isolates of Klebsiella pneumoniae (K. pneumoniae), particularly the extended-spectrum β-lactamase (ESBL) producing strains, are mostly associated with the failure of antibiotic therapy in nosocomial infections. The present work was designed to evaluate the impact of Mr. Trivedi’s biofield energy treatment on phenotypic and genotypic characteristics of K. pneumoniae. The strain of K. pneumoniae bearing ATCC 15380 (American Type Culture Collection) was procured from the Bangalore Genei, in sealed pack and divided into control and treated groups. Treated group was subjected to Mr. Trivedi’s biofield energy treatment and analyzed for the antimicrobial susceptibility, minimum inhibitory concentration (MIC), biochemical reactions, and biotyping using automated MicroScan Walk-Away® system. Further, the effect of biofield treatment was also evaluated using Random Amplified Polymorphic DNA (RAPD) in order to determine their epidemiological relatedness and genetic characteristics of biofield treated K. pneumoniae samples. The antimicrobial susceptibility results showed an improve sensitivity (i.e. from intermediate to susceptible) of ampicillin/sulbactam and chloramphenicol, while altered sensitivity of cephalothin (i.e. from susceptible to intermediate) was also reported as compared to the control sample. The MIC value showed two-fold decrease in MIC value of ampicillin/sulbactam (i.e. 16/8 to ≤8/4 μg/mL) and chloramphenicol (i.e. 16 to ≤ 8 μg/mL) as compared to the control. The cephalothin showed two-folds change (i.e. ≤ 8 to 16 μg/mL) in the MIC value as compared with the control. Biofield treatment showed 9.09% alterations in biochemical reactions followed by a change in biotype number (7774 4272) in the treated group with respect to the control (7774 4274). Genetic fingerprinting was performed on control and treated samples using RAPD-PCR biomarkers, which showed an average range of 11 to 15% of polymorphism among the treated samples with respect to the control. These results suggested that Mr. Trivedi’s biofield energy treatment has a significant impact on K. pneumoniae.
Pathogenic isolates of Klebsiella pneumoniae (K. pneumoniae), particularly the extended-spectrum β-lactamase (ESBL) producing strains, are mostly associated with the failure of antibiotic therapy in nosocomial infections. The present work was designed to evaluate the impact of Mr. Trivedi’s biofield energy treatment on phenotypic and genotypic characteristics of K. pneumoniae. The strain of K. pneumoniae bearing ATCC 15380 (American Type Culture Collection) was procured from the Bangalore Genei, in sealed pack and divided into control and treated groups. Treated group was subjected to Mr. Trivedi’s biofield energy treatment and analyzed for the antimicrobial susceptibility, minimum inhibitory concentration (MIC), biochemical reactions, and biotyping using automated MicroScan Walk-Away®system. Further, the effect of biofield treatment was also evaluated using Random Amplified Polymorphic DNA (RAPD) in order to determine their epidemiological relatedness and genetic characteristics of biofield treated K. pneumoniaesamples. The antimicrobial susceptibility results showed an improve sensitivity (i.e. from intermediate to susceptible) of ampicillin/sulbactam and chloramphenicol, while altered sensitivity of cephalothin (i.e. from susceptible to intermediate) was also reported as compared to the control sample. The MIC value showed two-fold decrease in MIC value of ampicillin/sulbactam (i.e. 16/8 to ≤8/4 μg/mL) and chloramphenicol (i.e. 16 to ≤ 8 μg/mL) as compared to the control. The cephalothin showed two-folds change (i.e. ≤ 8 to 16 μg/mL) in the MIC value as compared with the control. Biofield treatment showed 9.09% alterations in biochemical reactions followed by a change in biotype number (7774 4272) in the treated group with respect to the control (7774 4274). Genetic fingerprinting was performed on control and treated samples using RAPD-PCR biomarkers, which showed an average range of 11 to 15% of polymorphism among the treated samples with respect to the control. These results suggested that Mr. Trivedi’s biofield energy treatment has a significant impact on K. pneumoniae.
Klebsiella pneumoniae - fakultativ anaerob, qrammənfi çöpşəkilli Klebsiella cinsindən olan bakteriya növü, pnevmoniya törədicisi. Klebsella pneumoniae pnevmoniya törədicisi olmaqla yanaşı sidik-cinsiyyət sistemi infeksiyaları ilə də assosiyasiya olunur. Normada bu bakteriya insanın mədə-bağırsaq traktında və ağız boşluğu florasında məskunlaşmışdır. Normal insanlar üçün bir o qədər də təhlükəli sayılmasa da immun sistemi zəifləmiş yaxud zədələnmiş insanlarda təhlükəli iltihabi prossesin yaranmasına səbəb ola bilir. Nadir hallarda pnevmoniya törədicicsi olan klebsella pneumoniae ilk dəfə 1883 ci ildə alman mikrobioloqu Karl Fridlender (1847-1887) tərəfindən aşkar edilmişdir. Bu bakteriyanın törətdiyi ağciyərlərin iltihabı tibbdə Friedlender pnevmoniyası da adlandırılır. Karl Fridlender o zaman bu mikrobu lat. diplococcus adlandırır. Bir qədər sonra bakteriyanın 3 yarımnövü aşkar edilir:
Klebsiella pneumoniae és l'espècie de major rellevància clínica dins el gènere bacterià Klebsiella. Són bacteris gramnegatius de la família Enterobacteriaceae, que tenen un important paper com a causants de malalties infeccioses oportunistes. El gènere va ser anomenat així en honor a Edwin Klebs, un microbiòleg alemany de finals del segle XIX. El bacil també va ser descrit per Carl Friedländer, i durant molts anys va ser conegut com el bacil de Friedländer.
Aquest bacteri està implicada principalment en infeccions nosocomials. És l'agent causal d'infeccions del tracte urinari, pneumònies, sèpsia, infeccions de teixits tous, i infeccions de ferida quirúrgica. Són especialment susceptibles els pacients ingressats en unitats de cures intensives, nounats, i pacients amb MPOC, diabetis mellitus o alcohòlics. Avui dia també hi ha una forta teoria que la relaciona amb l'espondilitis anquilosant.[1]
Causa al voltant de l'1% de les pneumònies bacterianes i pot causar condensació hemorràgica extensa del pulmó. A més, de vegades provoca infecció de l'aparell urinari i bacterièmia a partir de lesions focals en pacients debilitats que pot posar fi a la vida del pacient. Algunes de les complicacions més freqüents són l'abscés pulmonar i l'empiema.
A la tinció de Gram són negatius (color fúcsia); l'assimilació i la fermentació de la lactosa es pot observar a l'agar MacConkey on les colònies són de color rosat clar i en el medi Kliger o TSI on són àcid/àcid, és a dir, fermentadors de la lactosa amb producció de gas. En la fermentació de l'acetona o prova de Voges Proskauer són positius. Finalment, les seves condicions òptimes de cultiu són, en agar nutritiu, a 37 °C, pH de 7.0, i una pressió osmòtica d'1 atm.
Klebsiella pneumoniae és l'espècie de major rellevància clínica dins el gènere bacterià Klebsiella. Són bacteris gramnegatius de la família Enterobacteriaceae, que tenen un important paper com a causants de malalties infeccioses oportunistes. El gènere va ser anomenat així en honor a Edwin Klebs, un microbiòleg alemany de finals del segle XIX. El bacil també va ser descrit per Carl Friedländer, i durant molts anys va ser conegut com el bacil de Friedländer.
Aquest bacteri està implicada principalment en infeccions nosocomials. És l'agent causal d'infeccions del tracte urinari, pneumònies, sèpsia, infeccions de teixits tous, i infeccions de ferida quirúrgica. Són especialment susceptibles els pacients ingressats en unitats de cures intensives, nounats, i pacients amb MPOC, diabetis mellitus o alcohòlics. Avui dia també hi ha una forta teoria que la relaciona amb l'espondilitis anquilosant.
Causa al voltant de l'1% de les pneumònies bacterianes i pot causar condensació hemorràgica extensa del pulmó. A més, de vegades provoca infecció de l'aparell urinari i bacterièmia a partir de lesions focals en pacients debilitats que pot posar fi a la vida del pacient. Algunes de les complicacions més freqüents són l'abscés pulmonar i l'empiema.
A la tinció de Gram són negatius (color fúcsia); l'assimilació i la fermentació de la lactosa es pot observar a l'agar MacConkey on les colònies són de color rosat clar i en el medi Kliger o TSI on són àcid/àcid, és a dir, fermentadors de la lactosa amb producció de gas. En la fermentació de l'acetona o prova de Voges Proskauer són positius. Finalment, les seves condicions òptimes de cultiu són, en agar nutritiu, a 37 °C, pH de 7.0, i una pressió osmòtica d'1 atm.
Klebsiella pneumoniae je gramnegativní, nepohyblivá, zapouzdřená, laktózu fermentující, fakultativně anaerobní tyčinkovitá bakterie tvořící součást běžné flóry v ústech, trávicím traktu a na kůži.[1] Je klinicky nejvýznamnějším členem rodu Klebsiella z čeledi Enterobacteriaceae. Je blízkou příbuznou bakterie K. oxytoca, od které se liší svou indol-negativitou a schopností růst jak na melezitóze, tak na 3-hydroxybutyrátu. Přirozeně se vyskytuje v půdě a zhruba 30 % kmenů dokáže za anaerobních podmínek fixovat dusík.[2] Její dusíkový fixační systém byl jakožto u volně žijícího diazotrofa důkladně studován.
Členové rodu Klebsiella na svém povrchu vykazují typicky dva druhy antigenů. První, antigen 0, je součástí lipopolysacharidu (LPS), který existuje v 9 varietách. Druhým je antigen K, kapsulární polysacharid s více než 80 varietami.[3] Oba přispívají k patogenitě a tvoří základ pro sérotypizaci.
Dánský vědec Hans Christian Gram (1853–1938) vyvinul v roce 1884 techniku dnes známou jako Gramovo barvení, aby od sebe odlišil K. pneumoniae a Streptococcus pneumoniae.
Klebsiellanese jméno podle německého bakteriologa Edwina Klebse (1834–1913).
Multirezistentní Klebsiella pneumoniae je při laboratorních testech in vivo ničena intraperitoneálním, intravenózním nebo intranasálním podáním fágů.[4]
K. pneumoniae může způsobovat pneumonii.
Výzkum prováděný na King's College v Londýně vyvozuje, že molekulární mimikry mezi HLA-B27 a dvěma povrchovými molekulami na bakterii Klebsiella jsou příčinou ankylozující spondylitidy.[5]
Infekce bakteriemi Klebsiella se obecně vyskytují hlavně u lidí s imunitním systémem oslabeným nevhodnou stravou (alkoholiků a diabetiků). Mnoho z těchto infekcí lidé získávají při hospitalizaci v nemocnici (nozokomiální infekce). Nejčastějším infekčním onemocněním způsobovaným bakteriemi Klebsiella mimo nemocnice je pneumonie.
Vyskytují se nové rezistentní kmeny K. pneumoniae, čím dál častěji jako nozokomiální infekce.[6]
Bakterie Klebsiella jsou druhým nejčastějším (po E. coli) původcem infekce močových cest u starších lidí. Jsou také oportunním patogenem u pacientů s chronickým onemocněním plic, střevním onemocněním, atrofií sliznice nosní a rhinoskleromem. Nejvýznamnějším zdrojem infekce je stolice, druhým pak kontakt s kontaminovanými nástroji.
Podle informací publikovaných European Center for Disease Prevention and Control (ECDC) je u K. pneumoniae antibiotická rezistence vážným problémem. V roce 2010 byla míra rezistence na antibiotika „poslední instance“ 15 %, zatímco o pět let dříve jen okolo 7 %. V některých evropských státech je rezistentních skoro 50 % kmenů. V řadě států EU je 15–50 % kmenů zjištěných u infekcí v krevním řečišti rezistentních na karbapenemy.[7]
V tomto článku byl použit překlad textu z článku Klebsiella pneumoniae na anglické Wikipedii.
Klebsiella pneumoniae je gramnegativní, nepohyblivá, zapouzdřená, laktózu fermentující, fakultativně anaerobní tyčinkovitá bakterie tvořící součást běžné flóry v ústech, trávicím traktu a na kůži. Je klinicky nejvýznamnějším členem rodu Klebsiella z čeledi Enterobacteriaceae. Je blízkou příbuznou bakterie K. oxytoca, od které se liší svou indol-negativitou a schopností růst jak na melezitóze, tak na 3-hydroxybutyrátu. Přirozeně se vyskytuje v půdě a zhruba 30 % kmenů dokáže za anaerobních podmínek fixovat dusík. Její dusíkový fixační systém byl jakožto u volně žijícího diazotrofa důkladně studován.
Členové rodu Klebsiella na svém povrchu vykazují typicky dva druhy antigenů. První, antigen 0, je součástí lipopolysacharidu (LPS), který existuje v 9 varietách. Druhým je antigen K, kapsulární polysacharid s více než 80 varietami. Oba přispívají k patogenitě a tvoří základ pro sérotypizaci.
Klebsiella pneumoniae ist ein fakultativ anaerobes, gramnegatives Stäbchenbakterium aus der Gattung Klebsiella, das in der Lage ist, den Zweifachzucker Lactose (Milchzucker) abzubauen. Außerdem kann es den in der Luft vorhandenen Stickstoff zu Ammoniak bzw. Ammonium reduzieren, dieser Stoffwechselweg wird als Stickstofffixierung bezeichnet. 1984 wurde die Art in drei Unterarten (Subspezies) aufgeteilt. Das Bakterium ist überall verbreitet. Beim Menschen gehört es zu den normalen Bewohnern des Darms. In anderen Körperregionen kann es jedoch als Krankheitserreger auftreten, auch bei Tieren. Das Genom des Bakterienstammes Klebsiella pneumoniae subsp. pneumoniae DSM 30104 wurde im Jahr 2012 vollständig sequenziert.
Unter den Vertretern der Gattung ist Klebsiella pneumoniae von besonderer medizinischer Bedeutung, für diese Art sind im Krankenhaus erworbene Lungenentzündungen (nosokomiale Pneumonien) und andere Infektionen typisch. Sie verfügt über mehrere Virulenzfaktoren und es sind multiresistente Bakterienstämme bekannt, d. h. sie sind gegen viele Antibiotika resistent, so dass die Arzneimittel bei einer Infektion mit diesen Bakterienstämmen nicht mehr wirken. Personen mit geschwächtem Immunsystem oder mit akuten Infektionen sind gefährdet, auch die Stärke der Kontamination kann entscheiden.
Die Zellen von Klebsiella pneumoniae erscheinen im lichtmikroskopischen Bild als kurze Stäbchen mit einer Länge von 1–2 µm und einer Breite von 0,5–0,8 µm. Sie liegen einzeln oder in Paaren vor und sind von einer Schleimkapsel (Glykokalyx) umgeben.[2] In der Gram-Färbung werden sie rosa bis rot angefärbt, sie sind gramnegativ. Wie für die Gattung Klebsiella typisch, sind sie nicht aktiv beweglich (motil), besitzen also keine Flagellen (Geißeln). Die Zelloberfläche ist jedoch mit Fimbrien besetzt.[2] Auf einem Nährboden gewachsene Bakterienkolonien weisen keine besondere Färbung auf, sie sind konvex erhaben, in der Aufsicht rund und mit einem Durchmesser von 3–4 mm eher groß, typisch ist ihr schleimiges Aussehen.[3] Dieses wird durch die Anhäufung extrazellulärer Polysaccharide verursacht, die zusammen mit dem vorhandenen Wasser einen Biofilm bilden.[2]
Wie bei den Vertretern der Enterobacteriaceae üblich, verlaufen der Katalase-Test positiv und der Oxidase-Test negativ.[4] Klebsiella pneumoniae ist fakultativ anaerob, d. h. sie kann mit oder ohne Sauerstoff wachsen. Sie ist in der Lage, das Disaccharid Lactose zu verwerten.[3] Weitere Informationen sind im Abschnitt Biochemische Nachweise zu finden.
Außerdem gehört sie zu den stickstofffixierenden Mikroorganismen, sie kann elementaren, molekularen Stickstoff (N2) zu Ammoniak (NH3) bzw. Ammonium (NH4+) reduzieren und damit biologisch verfügbar machen. Dies erfolgt mit Hilfe des Enzymkomplexes Nitrogenase in einem anoxischen Milieu, da der Enzymkomplex durch Sauerstoff inaktiviert wird. Klebsiella pneumoniae ist diazotroph, kann also mit N2 als Stickstoffquelle wachsen, um daraus zelleigene Stoffe wie Aminosäuren aufzubauen.[3]
Für die Kultivierung sind einfache Nährmedien geeignet, beispielsweise Casein-Soja-Pepton-Agar (CASO-Agar), auch auf Columbia-Blutagar lassen sich die Bakterien anzüchten.[5] Häufig werden Selektivnährmedien verwendet, die zur Isolierung und Unterscheidung von Vertretern der Enterobakterien geeignet sind, beispielsweise MacConkey-Agar und Eosin-Methylen-Blau-Agar (EMB), die beide Lactose enthalten. Für eine weitere Selektion wird ein Nährmedium empfohlen, das als Kohlenstoffquelle (organische Verbindung zur Energiegewinnung) nur Citrat und Inositol enthält, es basiert auf dem Simmons Citrat-Agar mit einem Zusatz von 1 % Inositol.[3] Klebsiella pneumoniae ist mesophil, optimales Wachstum erfolgt bei einer Temperatur von 30–37 °C, nach Inkubation über ein bis zwei Tage sind Kolonien sichtbar.[5] Wachstum erfolgt auch noch bei 41 °C, nicht jedoch bei 5 °C. Bakterienstämme, die aus medizinischem Untersuchungsmaterial isoliert wurden, wachsen meist optimal bei 37 °C, jedoch verlaufen verschiedene Nachweisreaktionen zur Identifizierung besser bei einer Inkubationstemperatur von 30 °C.[3]
Bestandteile der Bakterienzelle wirken als Antigene, bei Klebsiella sind dies 77 verschiedene K-Antigene (K verweist auf die Kapsel), sowie 9 somatische O-Antigene. Von diagnostischer Bedeutung sind die K-Antigene, durch serologische Untersuchung lassen sich die verschiedenen Serotypen unterscheiden, was u. a. bei der Aufklärung von epidemiologischen Zusammenhängen angewandt wird. Es gibt jedoch ebenfalls ein ELISA-Verfahren zum Nachweis der O-Antigene.[3] Die Bestimmung kann auch mit Hilfe genetischer Untersuchungen erfolgen.
Der GC-Gehalt, also der Anteil der Nukleinbasen Guanin und Cytosin in der Bakterien-DNA, liegt beim Bakterienstamm DSM 30104 (aus der Stammsammlung DSM Deutsche Sammlung von Mikroorganismen und Zellkulturen) bei 57,0 Molprozent.[6] DSM 30104 ist der Typusstamm der Subspezies Klebsiella pneumoniae subsp. pneumoniae und damit auch der Spezies, er wurde aus menschlichem Blut isoliert.[7] Das Genom wurde im Jahr 2012 vollständig sequenziert.[6]
Es liegt als ringförmigen Bakterienchromosom vor und weist eine Größe von 5.512 Kilobasenpaaren (kb) auf, was in etwa mit der Genomgröße von Escherichia coli vergleichbar ist. Es sind 5.425 codierende Gene vorhanden, außerdem wurden 77 tRNAs identifiziert. Die Gene wurden mit der Antibiotic Resistance Genes Database (ARDB, Antibiotikaresistenz-Gendatenbank) verglichen, es konnten 15 Gene identifiziert werden, die eine Resistenz vermitteln, u. a. für eine Klasse A Beta-Lactamase und eine Effluxpumpe. Zehn weitere Gene codieren für Genprodukte, die die β-Lactamase-Fähigkeiten des Bakteriums erweitern, darunter das als ampC bezeichnete Gen, welches für das als AmpC-Beta-Lactamase (in diesem Fall eine Cephalosporinase) bezeichnete Enzym codiert und das als gloB bezeichnete Gen, welches für eine als Metallo-β-Lactamase (in diesem Fall eine Carbapenemase) bezeichnete Enzym codiert.[6] Seitdem wurden über 4.200 Genome (bezogen auf das zirkuläre Bakterienchromosom) dieser Spezies sequenziert, außerdem 913 Annotationen von Plasmiden durchgeführt (Stand 2018).[8]
Plasmide tragen häufig die genetische Information für eine Antibiotikaresistenz (siehe unten) des Bakteriums, die Genprodukte sind Enzyme, die eine bestimmte chemische Struktur eines Antibiotikums verändern und dadurch die Wirkung des Arzneistoffes verhindern. Bei Klebsiella pneumoniae sind dies plasmidcodierte Beta-Lactamasen, wie die SHV-1, TEM-1, TEM-2 oder weitere ESBL (Extended Spectrum β-Lactamasen).[3] Seit Beginn des 21. Jahrhunderts beobachtet man auch Resistenzen gegen Carbapeneme, verursacht durch Carbapenemasen (carbapenem-hydrolyzing beta-lactamase), die nach dem produzierenden Bakterium als KPC (Klebsiella pneumoniae Carbapenemasen) bezeichnet werden, verschiedene Varianten werden KPC-1, KPC-2 oder KPC-3 genannt.[9] Die Besonderheit von Plasmiden ist, dass sie durch horizontalen Gentransfer zwischen verschiedenen Bakterienarten ausgetauscht werden und somit die Antibiotikaresistenz „übertragen“ wird. Ein klinischer Fallbericht der Übertragung eines Plasmids mit dem Resistenzgen blaKPC-3 von K. pneumoniae auf K. aerogenes ist dort im Artikel beschrieben.
Die Untersuchung der Nukleotidsequenz einzelner Gene ergab, dass die Art Klebsiella pneumoniae eine große Diversität aufweist. Weitere genetische Untersuchungen, beispielsweise eine Abwandlung des PCR-Verfahrens mit zufällig vervielfältigter polymorpher DNA (RAPD), bestätigen das Vorkommen von drei unterschiedlichen phylogenetischen Gruppen, die als KpI, KpII und KpIII bezeichnet werden. Sie sind nicht mit den drei Subspezies identisch.[3] Weiterführende genetische Untersuchungen in den letzten Jahren, wie Sequenzierung der 16S ribosomalen RNA (rRNA) und Multi-Locus Sequenzanalyse (MLSA) bestimmter Gene haben dazu geführt, die Vertreter der Gruppe KpII als Klebsiella quasipneumoniae zu klassifizieren bzw. die Stämme der phylogenetischen Gruppe KpIII als Klebsiella variicola.[10]
Die drei Subspezies von K. pneumoniae werden durch die Biostoffverordnung in Verbindung mit der TRBA (Technische Regeln für Biologische Arbeitsstoffe) 466 der Risikogruppe 2 zugeordnet. Bei K. pneumoniae subsp. pneumoniae und K. pneumoniae subsp. rhinoscleromatis findet sich außerdem die Bemerkung ht, sie zeigt an, dass das Bakterium pathogen für Mensch und Wirbeltiere ist, jedoch in der Regel keine Übertragung zwischen beiden Wirtsgruppen erfolgt.[11]
K. pneumoniae verfügt über mehrere Virulenzfaktoren. Die Kapsel (Glykokalyx) schützt vor Phagozytose durch die Phagozyten, Zellen des Immunsystems. Dabei stört sie das an der Abwehr von Mikroorganismen beteiligte Komplementsystem, indem dessen Aktivierung oder die Aufnahme bereits freigesetzter Polypeptide, wie C3b verhindert wird.[2] Adhäsine ermöglichen das Anheften an die Wirtszellen. Einige Adhäsine von K. pneumoniae wirken gleichzeitig als Hämagglutinine und sind den Fimbrien (Pili) zuzuordnen. Die Typ-1-Fimbrien führen bei Erythrozyten von Meerschweinchen zu einer sichtbaren Verklumpung (Agglutination), sie heften sich an humane Epithelzellen des Darms oder Epithelzellen der ableitenden Harnwege. K. pneumoniae-Isolate aus medizinischen Proben bilden mehr Typ-1-Fimbrien aus als Isolate aus Umweltproben.[3] Auch Typ-3-Fimbrien kommen vor, sie vermitteln die Anheftung der Bakterien an das pflanzliche Wurzelsystem, sowie beim Menschen an Endothelzellen, Epithelzellen der Lungenbläschen und der ableitenden Harnwege und an das Kollagen Typ V. Welche Rolle die Typ-3-Fimbrien bei der Infektion von Menschen spielen, ist noch Gegenstand der Forschung. Es wird vermutet, dass sie für die Kolonisation von invasiven medizinischen Geräten, die über eine längere Zeit im Körper verbleiben, verantwortlich sind.[3]
Die Lipopolysaccharide (LPS) der Äußeren Membran wirken als Antigene, die nach außen gerichteten Polysaccharidketten werden als O-Antigene bezeichnet (man vergleiche das bei den Salmonellen angewendete Kauffmann-White-Schema). K. pneumoniae besitzt neun verschiedene O-Antigene, wobei O1 am häufigsten vorkommt.[3] Die O-Antigene stören ebenfalls die Reaktionskaskade des Komplementsystems.[2] Außerdem ist O1 an der Nekrose von infiziertem Gewebe beteiligt.[3] Auch die bakteriellen Siderophore sind für die Pathogenität von Bedeutung. Sie dienen dazu, die Zellen mit für den Stoffwechsel essentiellen Eisen-Ionen zu versorgen, indem sie Fe3+-Ionen binden. K. pneumoniae bildet Enterobactin (Enterochelin), während nur einige Stämme zusätzlich noch Aerobactin produzieren. Bei den Serotypen K1 und K2 hat man ein Plasmid gefunden, auf dem die genetische Information für das Hydroxamat Aerobactin codiert ist. Werden diese Gene in einen Stamm ohne Plasmid mit Hilfe der Transformation übertragen, so weisen die transformierten Zellen eine um den Faktor 100 gesteigerte Virulenz auf. Auch Yersiniabactin, ein für Yersinia-Arten typisches Siderophor wird von einigen Stämmen gebildet.[3]
K. pneumoniae ist nah mit K. aerogenes (früher in die Gattung Enterobacter gestellt) und Enterobacter cloacae verwandt. Die Bakterien zeigen eine ausgesprochene Vielseitigkeit in Bezug auf die Verwertung diverser Kohlenhydrate und weisen bis auf wenige Ausnahmen gleiche biochemische Merkmale auf, wie beispielsweise die vorhandenen Enzyme und die daraus resultierenden Stoffwechseleigenschaften.[4]
Vertreter der Gattung Klebsiella führen als typische Gärung die 2,3-Butandiol-Gärung zur Energiegewinnung durch, im Voges-Proskauer-Test wird Acetoin, ein Zwischenprodukt der 2,3-Butandiol-Gärung, nachgewiesen. Vertreter der verwandten Gattungen Enterobacter und Klebsiella reagieren hierbei positiv. Dies gilt prinzipiell auch für K. pneumoniae,[2][3] allerdings zeigen die Subspezies oder einzelne Bakterienstämme unterschiedliche Reaktionen, also auch ein negatives Ergebnis im VP-Test. Der Typusstamm DSM 30104 ist im Gegensatz zur Beschreibung der Art VP-negativ (produziert also kein Acetoin aus Pyruvat), dafür zeigt er im Methylrot-Test ein positives Ergebnis, was typisch für Vertreter der gemischten Säuregärung ist.[5] Diese Unterschiede im physiologischen Phänotyp spiegeln die genetische Diversität der Bakterienart wider. Auch weitere biochemische Merkmale sind innerhalb der Art nicht eindeutig festzulegen. So ist der Indol-Test als Unterscheidungsmerkmal zwischen K. pneumoniae (Indol-negativ) und Klebsiella oxytoca (Indol-positiv) grundsätzlich geeignet, allerdings gibt es auch einige Indol-positive Stämme von K. pneumoniae.[3]
Anstatt das Bakterium nachzuweisen, beschränkt man sich oft auf die Bestimmung des Serotyps oder den Nachweis einzelner Virulenzfaktoren oder Resistenzgene. Die K- und O-Antigene können sowohl „konventionell“ serologisch (in der englischsprachigen Literatur als serotyping bezeichnet) wie seit Verbreitung der molekularbiologischen Methoden auch durch diese bestimmt werden, beispielsweise mit Hilfe der Multi-Locus Sequenzanalyse (MLSA). Durch Vergleich mit den zahlreichen sequenzierten Genomen der Art konnte gezeigt werden, dass bei Stämmen mit den Kapsel-Antigenen K1 oder K2 fast immer der Serotyp O1 auftritt. Die Serotypen K1 und K2 werden als hypervirulent angesehen.[12] Die Bestimmung der Kapsel-Antigene kann ebenfalls durch die Multiplex-PCR (es wird mehr als ein Genomabschnitt nachgewiesen) und die Pulsed-Field-Gelelektrophorese (PFGE) erfolgen.[13]
Die Identifizierung mit Hilfe der MALDI-TOF-Methode in Kombination mit Massenspektrometrie (MS) ist grundsätzlich geeignet, Klebsiella nachzuweisen, ist aber nicht immer zuverlässig in Bezug auf die Unterscheidung nah verwandter Gattungen, beispielsweise zu Raoultella. Die Spektren vieler gramnegativer Spezies, die zu den Enterobakterien gehören, zeigen eine große Übereinstimmung (Stand 2013), was die Identifizierung erschwert. Eine andere systematische Untersuchung von in einer flüssigen, bluthaltigen Nährlösung kultivierten Bakterien zeigte, dass insbesondere Bakterien mit einer Kapsel nicht korrekt identifiziert werden.[14] Hingegen kann beim Nachweis der Antibiotikaresistenz MALDI-TOF angewendet werden, um das Fehlen oder verringerte Vorhandensein von Proteinen in der äußeren Membran (englisch: outer membrane proteins, OMP) zu detektieren. Bei K. pneumoniae ist hier OmpK36 von Bedeutung, ein wichtiges Membranporin, durch das β-Lactam-Antibiotika in die Zelle gelangen. Bei resistenten Stämmen fehlt es oder wird in geringer Zahl ausgebildet.[14]
Der deutsche Mikrobiologe Carl Friedländer beschrieb diese Bakterien erstmals 1883 als Erreger einer seltenen Form der Lungenentzündung (Friedländer-Pneumonie). Er nannte sie damals noch „Diplococcus“. Kurz danach wurden die „Friedländer-Bakterien“ als „Bacterium pneumoniae crouposae“ Zopf 1885, „Hyalococcus pneumoniae“ Schroeter 1886 und „Bacillus pneumoniae“ (Schroeter 1886) Flügge 1886 beschrieben.[1]
1984 wurde die Art in drei Unterarten (Subspezies) aufgeteilt:[1]
Klebsiella pneumoniae (Schroeter 1886) Trevisan 1887 ist durch den Typusstamm ATCC 13883 (= CCUG 225 = CIP 82.91 = DSM 30104 = HAMBI 450 = IAM 14200 = IFO (NBRC) 14940 = JCM 1662 = LMG 2095 = NCTC 9633) definiert, nach der Unterteilung in drei Subspezies ist dies auch der Typusstamm für K. pneumoniae subsp. pneumoniae (Schroeter 1886) Ørskov 1984.[1][5]
Die Unterteilung in drei Subspezies beruht auf Merkmalen der Pathogenese und nicht auf der ausreichenden Unterscheidbarkeit der DNA-Sequenz. Durch genetische Untersuchungen wurde 2003 gezeigt, dass innerhalb der Art mindestens drei unterschiedliche phylogenetische Gruppen existieren, dies sind jedoch nicht die hier erwähnten Unterarten. Bei durch K. pneumoniae verursachten Lungenentzündungen sind zwar Stämme, die zur phylogenetischen Gruppe KpI gehören, vorherrschend, aber Vertreter der Gruppen KpII (K. quasipneumoniae) und KpIII (K. variicola) kommen ebenfalls vor.[3]
Klebsiella pneumoniae ist ubiquitär verbreitet. Ihre natürlichen Habitate sind Gewässer, Abwasser (besonders von Industrieanlagen der Papierherstellung und Fruchtverarbeitung), Erdboden und Pflanzen, dort insbesondere die Wurzeln. Die Bakterien heften sich mit Hilfe ihrer Fimbrien (vor allem der Typ-3-Fimbrien) an die Wurzelhaare der Rhizodermis, um dort Stickstoff zu fixieren. Die Stickstofffixierung erfolgt – anders als bei den Rhizobien (Knöllchenbakterien), die ausschließlich in Symbiose mit Pflanzen aus der Familie der Leguminosen leben – bei K. pneumoniae assoziativ mit verschiedenen höheren Pflanzen (Gefäßpflanzen), manchmal als Endophyt. So wurde K. pneumoniae auch von Blättern der Reispflanze, dem Gewebe der Halme von Zea mays (Mais) und verrottendem Holz isoliert. Bei Literaturangaben zum Vorkommen ist jedoch zu beachten, dass die Identifizierung nicht immer zuverlässig war, so dass vereinzelt Isolate auch anderen Klebsiella-Arten bzw. Vertretern der Gattung Raoultella (die früher zur Gattung Klebsiella gestellt waren) zuzurechnen sind.[3]
Das Bakterium besiedelt auch Tiere und Menschen, u. a. den Darm, K. pneumoniae wurde von zahlreichen Vertretern der Säugetiere und Insekten isoliert. Bei Stuten (weiblichen Hauspferden) kann sie eine Metritis, eine Entzündung der Gebärmutter, verursachen. Dies gilt für Stämme mit dem Kapselantigen K1, K2 und K5, durch die es zu Epidemien gekommen ist. Hingegen werden Stämme mit dem Kapselantigen K7 eher als opportunistische Erreger betrachtet. Neben dem menschlichen Darm, wo es zur Darmflora gehört, findet sich das Bakterium auch im Nasenrachenraum (Nasopharynx). Vor dem Auftreten multiresistenter Stämme wurde angenommen, dass die Besiedelung der Patienten oder des Krankenhauspersonals das Reservoir ist, durch das eine Infektion mit K. pneumoniae verursacht werden kann. Untersuchungen bei epidemischen Ausbrüchen in Krankenhäusern – also bei nosokomialen Infektionen – mit Hilfe serologischer (Bestimmung der K-Antigene) oder molekularbiologischer Methoden zeigen ein anderes Bild. Häufig lässt sich später ein bestimmter antibiotikaresistenter Klon identifizieren, der für die Infektionen verantwortlich war.[3] Sofern ein Screening der Patienten bei Aufnahme und in regelmäßigen Abständen im Hinblick auf die Besiedelung mit K. pneumoniae erfolgt, kann bewiesen werden, dass dieser Klon nicht ursprünglich bei dem Patienten zu finden war. Untersuchungen in italienischen Krankenhäusern ergaben 2012 die Verbreitung der multiresistenten Klone ST101, ST258 und ST512.[15]
In Israel wurde eine Symbiose zwischen dem Bakterium, Larven des Steppenrüsslers Conorhynchus pistor und der Pflanze Salsola inermis beobachtet. Die Larven des Rüsselkäfers leben in Lehmkokons an den Wurzeln der Pflanze, die Bakterien leben im Verdauungstrakt der Käferlarven. Die Ausscheidungen der Käferlarven versorgen die Pflanze mit Stickstoff. Es wurde beobachtet, dass der Befall von den Käferlarven eine positive Auswirkung auf die Pflanzen hat.[16]
Bei durch Klebsiella pneumoniae (Klebsiella pneumoniae subsp. pneumoniae) ausgelösten Erkrankungen handelt es sich häufig um Infektionen der ableitenden Harnwege (Harnwegsinfekt, HWI bzw. CTI als Abkürzung im englischen Sprachraum) oder Atemwege (Pneumonien).[2] Eine Pneumonie (auch die Friedländer-Pneumonie) wird hauptsächlich durch Serotypen mit dem K1-Antigen verursacht, daneben können auch K2, K3, K4, K5 und K6 beteiligt sein.[3] Zunehmend ist das Bakterium bekannt dafür, nosokomiale Pneumonien bei immuninkompetenten stationären Patienten auszulösen.
Nosokomiale Infektionen („Krankenhausinfektionen“) bei immunsupprimierten Patienten werden häufig durch Anwendung invasiver medizinischer Verfahren, z. B. beim Einführen von Kathetern oder der intensivmedizinischen Beatmung verursacht. Die nosokomial erworbenen Infektionskrankheiten umfassen neben Pneumonien ebenfalls Harnwegsinfekte, Wundinfektionen, Bakteriämien bis zur Sepsis sowie Cholezystitis.[2] Neben immunsupprimierten Patienten sind Neugeborene gefährdet, in den letzten Jahren kam es weltweit zu mehreren epidemischen Ausbrüchen in Neugeborenen-Intensivstationen, mit Todesfolgen.[17] Die klinische Bedeutung von (nosokomialen) Infektionen mit K. pneumoniae ist verknüpft mit der verbreiteten Multiresistenz des Bakteriums. K. pneumoniae gehört zu den fünf häufigsten Krankheitserregern der bakteriellen Sepsis (6,7 % der Fälle, 2006 veröffentlicht) bzw. der nosokomial erworbenen Pneumonie (10,1 % der Fälle, 2010 veröffentlicht).[9] Sie gehört zur sogenannten ESKAPE-Gruppe.
Ein aktueller Ansatz zur Unterscheidung der Klebsiella pneumoniae-Bakterienstämme im Hinblick auf ihre medizinische Relevanz, der auch durch genetische Untersuchungen bestätigt wird, ist die Einteilung in folgende drei Gruppen: Opportunistische, hypervirulente und multiresistente Stämme.[18] Die opportunistischen Erreger infizieren insbesondere Patienten mit einem geschwächten Immunsystem und sind typisch für nosokomiale Infektionen (siehe oben). Hypervirulente K. pneumoniae sind sogenannte community acquired (deutsch „ambulant erworbene“) Stämme, die gesunde Menschen außerhalb von Einrichtungen des Gesundheitswesens besiedeln bzw. infizieren. Sie verursachen schwere Infektionen, wie beispielsweise Pyogenen Leberabszess, Endophthalmitis (Infektion im Auge) und Meningitis (Hirnhautentzündung), diese werden seit den 1990er Jahren vermehrt aus Asien und den Pazifikanrainerstaaten gemeldet.[18] Insbesondere die Serotypen K1 und K2 werden als hypervirulent bezeichnet. Genetische Vergleichsuntersuchungen zeigen, dass bei ihnen besonders viele Virulenzfaktoren auftreten, u. a. eine große Anzahl an Siderophoren, die Bildung von Colibactin (schädigt die DNA) sowie das Regulatorgen rmpA (mucosity regulator, bezieht sich auf die Schleimkapsel).[12] Für multiresistente K. pneumoniae ist die Produktion von Carbapenemasen typisch, dadurch wird die Behandlung der Infektion zunehmend schwierig (vergleiche folgende Abschnitte).
Es gibt Studien,[19] die darauf hinweisen, dass durch natürliche Abwehrreaktionen gebildete und gegen Klebsiella pneumoniae gerichtete IgA-Antikörper mit Strukturen des humanen Zelloberflächenproteins HLA-B27 kreuzreagieren. HLA-B27 reguliert wichtige Funktionen des menschlichen Immunsystems. Klebsiella pneumoniae steht im Verdacht, über diesen Mechanismus auch Autoimmunreaktionen wie z. B. Spondylitis ankylosans (Morbus Bechterew) auszulösen.
K. pneumoniae verfügt über eine natürliche Antibiotikaresistenz gegen Benzylpenicillin, Aminopenicilline (z. B. Ampicillin und Amoxicillin) und Carboxypenicilline (z. B. Carbenicillin und Ticarcillin), allesamt β-Lactam-Antibiotika. Die Resistenz beruht auf der im Bakterienchromosom codierten Klasse A Beta-Lactamase. Zusätzlich dazu auftretende, erworbene Resistenzen sind häufig durch plasmidcodierte ESBL (Extended Spectrum β-Lactamasen) wie die SHV-1, TEM-1 oder TEM-2 bedingt (siehe Abschnitt Genetik). Dadurch ist K. pneumoniae resistent gegen weitere Penicilline sowie Cephalosporine der 3. Generation (z. B. Cefotaxim und Ceftazidim). Ende der 1990er waren in den USA 24 % der K. pneumoniae-Stämme resistent gegen Ceftazidim.[3] An dem deutschen epidemiologische Überwachungsprogramm Surveillance der Antibiotika-Anwendung und der bakteriellen Resistenzen auf Intensivstationen (SARI) beteiligte Intensivstationen meldeten einen Anstieg der Resistenz gegenüber Cephalosporinen der 3. Generation von 2,2 % (2000) auf 16,8 % (2010).[9] Seit Beginn des 21. Jahrhunderts beobachtet man auch Resistenzen gegen Carbapeneme, verursacht durch Carbapenemasen (eine weitere Gruppe der β-Lactamasen), die nach dem produzierenden Bakterium als KPC (Klebsiella pneumoniae Carbapenemasen) bezeichnet werden (vergleiche nächster Abschnitt).
Erstmals 2001 wurde an einen bestimmten Klebsiella pneumoniae-Stamm die Bildung einer Carbapenemase (carbapenem-hydrolyzing beta-lactamase) beobachtet: Diese bewirkt eine Resistenz der Klebsiellen gegenüber bestimmten Antibiotika, den Carbapenemen. Zu diesen gehören z. B. die Arzneistoffe Imipenem und Meropenem. Die Aktivität der Carbapenemase wird jedoch in Gegenwart von Clavulansäure unterdrückt. Der untersuchte carbapenemresistente Klebsiella pneumoniae-Stamm (carbapenem-resistant Klebsiella pneumoniae, CRKP) „1534“ zeigte weiterhin Resistenz gegen alle Cephalosporine und Aztreonam und ist damit weitgehend unempfindlich gegen viele moderne Antibiotika.[20] Es sind verschiedene Varianten der Klebsiella pneumoniae Carbapenemasen bekannt, wie etwa KPC-1, KPC-2 und KPC-3.[21][22]
Carbapenemase-produzierende Enterobacteriaceae (CPE) werden in Deutschland als 3MRGN oder 4MRGN (multiresistente gramnegative Bakterien) klassifiziert.[9] In der Erregergruppe CPE ist K. pneumoniae überproportional vertreten, durch horizontalen Gentransfer sind die KPC jedoch auch bei verwandten Arten der Enterobakterien anzutreffen, beispielsweise bei Escherichia coli, Serratia marcescens, Enterobacter cloacae, Citrobacter freundii sowie bei K. oxytoca und K. aerogenes.[15] Da meist bei Auftreten einer ESBL oder einer Carbapenemase auch eine Resistenz gegen Fluorchinolone auftritt, sind diese Stämme gegenüber allen vier im MRGN-System definierten Antibiotikaklassen resistent und werden als 4MRGN bezeichnet.[9] Das Robert Koch-Institut (RKI) berichtete 2012 über den ersten Fall aus Deutschland, bei dem aus der Operationswunde an der Hüfte eines Patienten ein multiresistenter Stamm isoliert wurde. Das Antibiogramm bestätigte das Isolat als 4MRGN, zusätzlich wurde auch eine Resistenz gegenüber Colistin nachgewiesen, das in solchen Fällen eigentlich als Reserveantibiotikum verwendet wird.[23]
2012 warnte die beim RKI eingerichtete KRINKO (Kommission für Krankenhaushygiene und Infektionsprävention), „(…) dass sich Deutschland gerade am Beginn einer Entwicklung befindet, bei der es zur Zunahme Carbapenem-resistenter K. pneumoniae-Stämme kommt.“[9] International betrachtet sind CPE schon weit verbreitet oder zumindest ein vermehrt auftretendes Problem. Dies zeigt beispielsweise das international agierende Überwachungssystem European survey on carbapenemase-producing Enterobacteriaceae (EuSCAPE). An diesem Surveillance-System sind die 28 Mitgliedstaaten der Europäischen Union, sieben (potenzielle) Beitrittskandidaten, Island, Norwegen und Israel beteiligt. Ein 2013 veröffentlichter Zwischenbericht gibt an, dass 29 dieser 38 Staaten ein nationales Surveillance-System für Carbapenemase-produzierende Enterobacteriaceae (CPE) unterhalten, in allen nationalen Programmen wird auch Klebsiella pneumoniae überwacht. 33 der „nationalen Experten“ gaben dabei an, dass in ihrem Land K. pneumoniae die Bakterienart ist, die am häufigsten unter den CPE nachgewiesen wird.[24]
Im Washoe County, Nevada, USA wurde ein multiresistenter Klebsiella pneumoniae-Stamm an einer Patientin entdeckt, die zuvor in Indien mehrere Krankenhausaufenthalte hatte. Das Bakterium war gegen alle zugelassenen Antibiotika resistent. Im Labor zeigte einzig Fosfomycin eine Wirkung, das allerdings nur oral eingenommen werden konnte, da es in den USA keine Zulassung für eine intravenöse Behandlung hatte, wie sie die Patientin benötigte. Die Patientin verstarb, nachdem die Verabreichung aller 26 in den USA zugelassenen Antibiotika, u. a. auch Fosfomycin in oraler Form, keine Wirkung erzielte.[25] Im Mai 2017 fand man im Universitätsklinikum Frankfurt, dem größten Krankenhaus Hessens, bei fünf Patienten den Erreger Klebsiella pneumoniae 4MRGN.[26] 2011 und 2012 gab es im Klinikum Bremen-Mitte mehrere Todesfälle auf der Neugeborenen-Intensivstation durch multiresistente Stämme.[27]
Klebsiella pneumoniae ist ein fakultativ anaerobes, gramnegatives Stäbchenbakterium aus der Gattung Klebsiella, das in der Lage ist, den Zweifachzucker Lactose (Milchzucker) abzubauen. Außerdem kann es den in der Luft vorhandenen Stickstoff zu Ammoniak bzw. Ammonium reduzieren, dieser Stoffwechselweg wird als Stickstofffixierung bezeichnet. 1984 wurde die Art in drei Unterarten (Subspezies) aufgeteilt. Das Bakterium ist überall verbreitet. Beim Menschen gehört es zu den normalen Bewohnern des Darms. In anderen Körperregionen kann es jedoch als Krankheitserreger auftreten, auch bei Tieren. Das Genom des Bakterienstammes Klebsiella pneumoniae subsp. pneumoniae DSM 30104 wurde im Jahr 2012 vollständig sequenziert.
Unter den Vertretern der Gattung ist Klebsiella pneumoniae von besonderer medizinischer Bedeutung, für diese Art sind im Krankenhaus erworbene Lungenentzündungen (nosokomiale Pneumonien) und andere Infektionen typisch. Sie verfügt über mehrere Virulenzfaktoren und es sind multiresistente Bakterienstämme bekannt, d. h. sie sind gegen viele Antibiotika resistent, so dass die Arzneimittel bei einer Infektion mit diesen Bakterienstämmen nicht mehr wirken. Personen mit geschwächtem Immunsystem oder mit akuten Infektionen sind gefährdet, auch die Stärke der Kontamination kann entscheiden.
Klebsijela (lat. Klebsiella pneumoniae) je rod štapićastih bakterija s kapsulom. To je Gram-negativna, nepokretna, fakultativno anaerobna, bakterija koja fermentira laktozu. Javlja se kao fermentor sluzave laktoze na MacConkey agaru. Rod je imenovan po njemačkom patologu Edwinu Klebsu. Ljudi se najčešće zaraze bakterijama vrste Klebsiella pneumoniae.
Klebsijela se lahko nađe u normalnoj "flori" usta, kože i crijeva,[1] gdje može izazvati destruktivne promjene u ljudskim i životinjskim plućima, ako atmosferski (inhalacijski), uđe u alveole (pluća) što izaziva pojavu krvavog ispljuvka. U kliničkim uslovima, od svih pripadnika porodice Enterobacteriaceae, najznačajniji je roda Klebsiella. U ljudskim kliničkim uzorcima, pored Klebsiella pneumoniae pojavljuju se i Klebsiella oxytoca i Klebsiella rhinoscleromatis. U posljednjih nekoliko godina, vrste roda Klebsiella su postale važan patogen bolničkih infekcija.
Javlja se prirodno u tlu, a oko 30% sojeva može fiksirati dušik, u anaerobnim uslovima.[2] Mnogo je proučavana kao slobodno živeći diazotrof, zbog fiksacijskog sistema dušika, kao i zbog poljoprivrednih interesa, jer je uočeno da Klebsiella pneumoniae povećava prinos u takvim uslovima.[3]
Predstavnici roda i Klebsiella obično ispoljavaju dvije vrste antigena na površinama ćelija. Prvi, O antigen, sastavni je dio lipopolisaharida (LPS), od kojih postoji 9 varijanti. Drugi je K antigen, kapsulski polisaharid, s više od 80 varijanti.[4] Oba doprinose patogenosti i čine osnovu za serogrupisanje.
Klebsiella pneumoniae usko je srodna vrsti Klebsiella oxytoca, koja se prepoznaje po negativnom indol-testu i sposobnosti da raste na melezitozi, ali ne i 3-hidroksibutiratu.
Ranije su zarazi klebsijelom pretežno bili podložni alkoholičari, ali danas je najčešća pri medicinskim tretmanima u bolnicama i ustanovama za dugotrajnu njegu. Izaziva upalu pluća i mokraćnih puteva, infekcije u trbušnoj šupljini, hirurškim ranama, u koži i potkožnom tkivu i drugdje.
Infekcije klabsijelom uočavaju se uglavnom kod ljudi s oslabljenim imunim sistemom. Ova iscrpljujuća bolest najčešċe pogađa muškarce srednje dobi i starije. Vjeruje se da ova kategorija pacijenata ima poremećaj respiratorne odbrane domaćina, koja se pojavljuje i kod osoba sa: dijabetesom, alkoholičarskim problemom, om, sa bolestima jetre, hroničnom opstruktivnom bolešċu pluća, te one na terapiji glukokortikoidima, zbog zatajenja bubrega i određene izloženosti na radu (kao što su radnici u mljevaonicama papira). Mnoge od ovih zaraza se dobijaju kada je osoba u bolnici zbog nekih drugih zdravstvrnih problema, te tokom boravka tamo oboli od (bolničke infekcije). Najznačajniji izvor zaraze pacijenta je izmet, kao i kontakt sa zaraženim instrumentima.
Najčešća bolest koja je uzrokovana bakterijama roda Klebsiella izvan bolnice je pneumonija, obično u obliku bronhopneumonije i bronhitisa. Ovi pacijenti imaju povećanu podložnost za razvoj plućnog apscesa, kavitacije, empijema i pleuralne priraslice. Smrtnost kod ove bolesti je oko 50%, čak i sa antimikrobnom terapijom. Stopa smrtnosti može biti gotovo 100% za alkoholičare i bolesnike sa bakteremijom.
Osim upale pluća, klebsijela također može uzrokovati infekcije u urinarnom traktu, donjem žučnom traktu i mjestima hirurških rana. U ovu grupu kliničkih bolesti spadaju: upala pluća, tromboflebitis, infekcije urinarnog trakta, holecistitis, dijareju, upale gornjih respiratornih puteva, infekcije rana, osteomijelitis, meningitis, bakterijemija i septisemija. Za pacijente sa invazivnim uređajima u tijelu, postoji povećan rizik od infekcije tim putem. To su naprimjer, uređaji za njegu novorođenčadi, oprema za podršku disanja i urinarnni kateteri. Upotreba antibiotika također može biti činilac koji povećava rizik od bolničkih infekcija bakterijom klebsijela. Ulazak bakterija u krv može izazvati sepsu i sepsni šok.
Provedena istraživanja na Kraljevskom koledžu u Londonu su pokazala upletenost molekulske mimikrije između HLA-B27 i dvije površinske molekule klepsijele kao uzrok ankiloznog spondilitisa.[5]
Klebsiella se rangira kao druga, odmah iza Escherichia coli, kao uzročnik urinarnih infekcija kod starijih ljudi. Ona je oportunistički patogen za pacijente s hroničnim plućnim bolestima, patogen crijevne i nosne sluznice sa atrofijom i rinoskleromom.
Liječenje ovih infekcija otežava činjenica da su se pojavili novi sojevi Klesiella pneumoniae koji su otporni na antibiotike.[6]
Organizmi koji pripadaju rodu Klebsiella otporni su često na više antibiotika. Nova istraživanja ukazuju da su plazmidi glavni izvor gena koji određuju tu otpornost.[7] Vrste roda Klebsiella sa sposobnošću da proizvedu prošireni spektar beta-laktamaza (ESBL) otporne su na mnoge klase antibiotika. Najčešće su otpornosti na aminoglikozide, fluorohinolone, tetracikline, hloramfenikole i trimetoprim / sulfametoksazole.[8]
Infekcija s Enterobacteriaceae koje su otporne karbapenem (CRE) ili Enterobacteriaceae koje proizvode karbapenemazu, pojavljuju se kao važan izazov u postavkama zdravstvene zaštite.[9] Jedna od mnogih otpornih bakterija na karbapenem, proteklih 10 godina ispoljava progresivno povećanje učestalosti infekcija u svijetu. Međutim, ovaj novi soj u nastajanju bolničkih patogena je postao poznat po incidentu u Izraelu koji je započeo 2006. godine u okviru tamošnjeg zdravstvenog sistema.[10] U SAD-a, prvo se pojavila u Sjevernoj Karolini 1996. godine,[11] a od tada je otkrivena u 41 državi i[12] liječi se rutinski u nekim bolnicama u New Yorku i New Jerseyu. To je sada najčešća otporna vrsta bakterija u Sjedinjenim Američkim Državama.
Klebsijela koja je otporna na karbapenem, otporna je i na gotovo sva raspoloživa antimikrobna sredstva, a izaziva infekcije sa visokom stopom smrtnosti. Posebno je to slučaj među osobama s produženim ležanjem u bolnici, kritično bolesnih i izloženih invazivnim uređajima (naprimjer, ventilatori ili centralni venski kateter). Zabrinutost je povećana time što se karbapenem često koristi kao lijek u krajnjoj nuždi, kada se bori protiv otpornih sojeva bakterija. Nove tihe mutacije mogu dovesti do infekcije za koje zdravstveni radnici mogu vrlo malo učiniti, čak i kod liječenja pacijenata koji imaju otporne organizme.
Brojni su mehanizmi koji izazivaju otpornost na karbapenem u Enterobacteriaceae. Tu spadaju hiperprodukcija AmpC beta-laktamaza sa spoljnomembranskom porinskom mutacijom, CTX-M prošireni spektar beta-laktamaza s porinskom mutacijom ili isticanje lijeka i proizvodnja karbapenemaze. Najvažniji mehanizam otporosti je proizvodnja enzima karbapenemaza blakpc. Gen koji kodira ovaj enzim nalazi se na pokretnom dijelu genetskog materijala (transpozonu. On je posebno uključeni transpozon i označava se sa Tn4401), što povećava rizik od širenja. Otporne bakterije je teško otkriti, jer neki sojevi sa blakpc imaju minimalne inhibicijske koncentracije koje su povišene, ali i dalje u osjetljivom opsegu za karbapeneme. Budući da su ovi sojevi podložni karbapenemu, oni nisu identifikovani kao moguċi klinički ili kontrolni infekcijski faktori rizika, koristeći standardne smjernice za testiranje osjetljivosti. U uslovima bolničkog liječenja, rezervoari za prijenos bakterije su pacijenti sa neprepoznatim otpornim sojevima tokom bolničkih epidemija.
Obim i prevalencija otpornosti enterobakterija na karbapenem u okruženju je trenutno nepoznata. Stopa smrtnosti je također nepoznata, ali se sumnja da je u rasponu od 12,5% do 44%. Vjerovatnoća pojave epidemije ili pandemije u budućnosti i dalje je neizvjesna. Centar za kontrolu i sprečavanje bolesti objavio je smjernice za agresivnu kontrolu infekcije otpornih sojeva klebsijele:
Jedan poseban primjer ovakve politike zbio se u Izraelu, 2007.[14] Ova politika je imala period intervencije od aprila 2007. godine do maja 2008., sa nacionalnim širenjem otpornosti (koje je vrhunac dostiglo u martu 2007. godine na 55,5 slučajeva na 100.000 pacijentskih dana) i potrebe da se napravi nacionalni plan liječenja. Intervencija podrazumijevala fizičko odvajanje svih prenosnika i imenovanje radne grupe za nadzor efikasnosti izolacije, pomno praćenje bolnica i intervencije kada je to potrebno. Nakon što je ostvaren plan terapije (u maju 2008. godine), broj slučajeva u odnosu 100.000 pacijenata/broj dana smanjen je na 11,7. Plan je bio efikasan zbog strogih bolničkih usklađenosti, pri čemu je svaka imala detaljnu dokumentaciju svih nosilaca na antibiotike otpornih enterobakterija. U stvari, za svaki rast za 10%, učestalost slučajeva na 100.000 pacijenata/broj dana smanjena je za 0,6. Dakle, zadržavanje na nacionalnoj razini zahtijeva intervenciju pogođene zemlje.
U Sjedinjenim Američkim Državama, nadležne agencije preporučuju otkrivanje otpornosti na karbapenem ili proizvodnje karbapenemaze samo za Klebsiella spp. i Escherichia coli: ovo olakšava obavljanje testova u mikrobiološkoj laboratoriji, bez upotrebe molekulskih metoda, a ovi organizmi predstavljaju većinu otpornih bakterija nađenih u Sjedinjenim Američkim Državama. Efektivne procedure sterilizacije i dekontaminacije su posebno važne da bi stopa infekcije ovih sojeva otpornih na antibiotike bila što je moguće manja.
U Bosni i Hercegovini, obična klebsijela ("divlji tip") redovno se nalaze u ustima i probavnom traktu kod zdravih ljudi. Međutim, veliku zabrinutost izazvale su informacije o smrtnim slučajevima 10 prijevremeno rođenih beba na Univerzitetskoj kliničkoj bolnici u Mostaru, tokom 2016. godine. Nakon toga, u prvih deset mjeseci 2016., u ovoj bolnici su preminule još četiri bebe. Uzročnik smrti beba bio je zvanično "nepoznat", sve dok Federalna uprava za inspekcijske poslove nije, pregledom medicinske dokumentacije i hemokultura, ustanovila da je 11 (od ukupno 14) umrlih beba bilo zaraženo klebsijelom. Međutim, ostalo je nepoznato da li je Klabsiella pneumoniae, koja je otporna na antibiotike, bila odlučujući faktor u visokoj smrtmosti mostarske nedonoščadi.[15][16][17][18]
Kao i kod mnogih bakterija, preporučeni tretman se mijenjao kako su organizmi razvijali otpornost. Izbor određenog antimikrobnog agensa ili zastupnika zavisi o lokalnim obrascima osjetljivosti i zaraženom dijelu tijela. Za pacijente s teškim infekcijama, razborit pristup je korištenje početnog kratkog kursa (48-72 sata) kombinovane terapije, a zatim prelazak na određenu monoterapiju, kada je jednom obrazac podložnosti poznat za određenog pacijenta.
Ako određena Klebsiella kod liječenog pacijenta ne pokazuje otpornost na antibiotike, tada antibiotici koji se koriste za liječenje takvih izolata su: ampicilin / sulbaktam, piperacilin / tazobaktamom, ticarcillin / klavulonska kiselina, ceftazidim, cefepima, levofloksacin, norfloksacin, gatifloksacina, moksifloksacin, meropenem i ertapenem. Postoje tvrdnje da meropenem omogućava najbolje bakterijsko čišćenje.
Upotreba antibiotika obično nije dovoljna. Hirurško čišćenje (obično interventna radiološka drenaža) često je potrebna nakon što je pacijent počeo liječenje pomoću antimikrobnih agenasa.
Višestruko otporni Klebsiella pneumoniae sojevi se ubijaju in vivo, intraperitonealnom, intravenskom ili intranazalnom administracijom faga, u laboratorijskim testovima.[19] Iako je ovaj tretman dostupan već neko vrijeme, ne zna se da li veća opasnost prijeti od bakterijske ili od fagne otpornosti na antibiotike. Otpornost na fagne može uzrokovati porast broja mikroba u okruženju, kao i među ljudima. To je razlog zašto se fagna terapija koristi samo u kombinaciji s antibioticima, kako bi dopunil aktivnosti faga, umjesto da ih zamijene u potpunosti.[20]
Da bi se spriječilo širenje infekcija benterobakterija roda Klebsiella između pacijenata, zdravstveni radnici moraju slijediti posebne mjere opreza radi kontrole infekcije.[21] U te mjere spadaju strogo pridržavanje higijene ruku i opreme, kao što su odjeća i rukavice kada uđu u prostorije u kojima su pacijenti oboljeli od infekcije klebsijelom. Zdravstvene ustanove moraju slijediti stroge procedure čišćenja radi sprečavanja širenja bakterija roda Klebsiella.
Za sprečavanje širenja infekcije, pacijenti također trebaju vrlo često da peru svoje ruke:
Klebsijela (lat. Klebsiella pneumoniae) je rod štapićastih bakterija s kapsulom. To je Gram-negativna, nepokretna, fakultativno anaerobna, bakterija koja fermentira laktozu. Javlja se kao fermentor sluzave laktoze na MacConkey agaru. Rod je imenovan po njemačkom patologu Edwinu Klebsu. Ljudi se najčešće zaraze bakterijama vrste Klebsiella pneumoniae.
Klebsijela se lahko nađe u normalnoj "flori" usta, kože i crijeva, gdje može izazvati destruktivne promjene u ljudskim i životinjskim plućima, ako atmosferski (inhalacijski), uđe u alveole (pluća) što izaziva pojavu krvavog ispljuvka. U kliničkim uslovima, od svih pripadnika porodice Enterobacteriaceae, najznačajniji je roda Klebsiella. U ljudskim kliničkim uzorcima, pored Klebsiella pneumoniae pojavljuju se i Klebsiella oxytoca i Klebsiella rhinoscleromatis. U posljednjih nekoliko godina, vrste roda Klebsiella su postale važan patogen bolničkih infekcija.
Javlja se prirodno u tlu, a oko 30% sojeva može fiksirati dušik, u anaerobnim uslovima. Mnogo je proučavana kao slobodno živeći diazotrof, zbog fiksacijskog sistema dušika, kao i zbog poljoprivrednih interesa, jer je uočeno da Klebsiella pneumoniae povećava prinos u takvim uslovima.
Predstavnici roda i Klebsiella obično ispoljavaju dvije vrste antigena na površinama ćelija. Prvi, O antigen, sastavni je dio lipopolisaharida (LPS), od kojih postoji 9 varijanti. Drugi je K antigen, kapsulski polisaharid, s više od 80 varijanti. Oba doprinose patogenosti i čine osnovu za serogrupisanje.
Klebsiella pneumoniae usko je srodna vrsti Klebsiella oxytoca, koja se prepoznaje po negativnom indol-testu i sposobnosti da raste na melezitozi, ali ne i 3-hidroksibutiratu.
Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium. It appears as a mucoid lactose fermenter on MacConkey agar.
Although found in the normal flora of the mouth, skin, and intestines,[1] it can cause destructive changes to human and animal lungs if aspirated, specifically to the alveoli resulting in bloody, brownish or yellow colored jelly-like sputum. In the clinical setting, it is the most significant member of the genus Klebsiella of the Enterobacteriaceae. K. oxytoca and K. rhinoscleromatis have also been demonstrated in human clinical specimens. In recent years, Klebsiella species have become important pathogens in nosocomial infections.
It naturally occurs in the soil, and about 30% of strains can fix nitrogen in anaerobic conditions.[2] As a free-living diazotroph, its nitrogen-fixation system has been much-studied, and is of agricultural interest, as K. pneumoniae has been demonstrated to increase crop yields in agricultural conditions.[3]
It is closely related to K. oxytoca from which it is distinguished by being indole-negative and by its ability to grow on melezitose but not 3-hydroxybutyrate.
The genus Klebsiella was named after the German microbiologist Edwin Klebs (1834–1913). It is also known as Friedlander's bacillum in honor of Carl Friedländer, a German pathologist, who proposed that this bacterium was the etiological factor for the pneumonia seen especially in immunocompromised individuals such as people with chronic diseases or alcoholics.
Community-acquired pneumonia caused by Klebsiella pneumoniae may occasionally be called Friedländer's pneumonia.[4]
Illness most commonly affects middle-aged and older men more often than women with debilitating diseases. This patient population is believed to have impaired respiratory host defenses, including persons with diabetes, alcoholism, malignancy, liver disease, chronic obstructive pulmonary diseases, glucocorticoid therapy, kidney failure, and certain occupational exposures (such as papermill workers). Many of these infections are obtained when a person is in the hospital for some other reason (a nosocomial infection).
In addition to pneumonia, Klebsiella can also cause infections in the urinary tract, lower biliary tract, and surgical wound sites. The range of clinical diseases includes pneumonia, thrombophlebitis, urinary tract infection, cholecystitis, diarrhea, upper respiratory tract infection, wound infection, osteomyelitis, meningitis, and bacteremia, and sepsis. For patients with an invasive device in their bodies, contamination of the device becomes a risk; neonatal ward devices, respiratory support equipment, and urinary catheters put patients at increased risk. Also, the use of antibiotics can be a factor that increases the risk of nosocomial infection with Klebsiella bacteria. Sepsis and septic shock can follow entry of the bacteria into the blood.
Research conducted at King's College, London has implicated molecular mimicry between HLA-B27 and two Klebsiella surface molecules as the cause of ankylosing spondylitis.[5]
Klebsiella ranks second to E. coli for urinary tract infections in older people.[6] It is also an opportunistic pathogen for patients with chronic pulmonary disease, enteric pathogenicity, nasal mucosa atrophy, and rhinoscleroma. New antibiotic-resistant strains of K. pneumoniae are appearing.[7]
The most common condition caused by Klebsiella bacteria outside the hospital is pneumonia, typically in the form of bronchopneumonia and also bronchitis. These patients have an increased tendency to develop lung abscesses, cavitation, empyema, and pleural adhesions. It has a death rate around 50%, even with antimicrobial therapy. [8]
It is typically due to aspiration and alcoholism may be a risk factor, though it is also commonly implicated in hospital-acquired urinary tract infections, and COPD (chronic obstructive pulmonary disease) individuals.[9][10] In terms of the pathophysiology of Klebsiella pneumonia the neutrophil myeloperoxidase defense against K. pneumoniae is often seen. Oxidative inactivation of elastase is involved, while LBP helps transfer bacteria cell wall elements to the cells.[11][12]
Individuals with Klebsiella pneumoniae tend to cough up a characteristic sputum, as well as having fever, nausea, tachycardia, and vomiting. Klebsiella pneumoniae tends to affect people with underlying conditions, such as alcoholism.[9]
In terms of the diagnosis of Klebsiella pneumoniae the following can be done to determine if the individual has this infection, with the addition of susceptibility testing to identify drug-resistant organisms:[11][9]
Treatment for Klebsiella pneumoniae is by antibiotics such as aminoglycosides, pipercillin tazobactam, and cephalosporins, the choice depending upon antibiotic susceptibility testing, the person's health condition, medical history and severity of the disease.[10][13]
Klebsiella possesses beta-lactamase giving it resistance to ampicillin, many strains have acquired an extended-spectrum beta-lactamase with additional resistance to carbenicillin, amoxicillin, and ceftazidime. The bacteria remain susceptible to aminoglycosides and some cephalosporins, and varying degrees of inhibition of the beta-lactamase with clavulanic acid have been reported. Infections due to multidrug-resistant gram-negative pathogens in the ICU have invoked the re-emergence of colistin. However, colistin-resistant strains of K. pneumoniae have been reported in ICUs.[11][14][15][16] In 2009, strains of K. pneumoniae with gene called New Delhi metallo-beta-lactamase ( NDM-1) that even gives resistance against intravenous antibiotic carbapenem, were discovered in India and Pakistan. Klebsiella cases in Taiwan have shown abnormal toxicity, causing liver abscesses in people with diabetes mellitus (DM); treatment consists of third generation cephalosporins.
Hypervirulent (hvKp) is a rather recent K pneumoniae variant that is significantly more virulent than classical K. pneumoniae (cKp). While cKp is an opportunistic pathogen responsible for nosocomial infections that usually affect immunocompromised patients, hvKp is clinically more concerning since it also causes disease in healthy individuals and can infect virtually every site of the body. The genetic traits that lead to this pathotype are included in a large virulence plasmid and potentially on additional conjugative elements.[17]
These newly identified strains were described to overproduce capsule components and siderophores for iron acquisition, among other factors.[18] Although initial studies showed that hvKp is rather susceptible to antibiotic treatment, it has been recently shown that such strains can acquire resistance plasmids and become multiresistant to a variety of antibiotics.[18][19][20]
It is originated from Asia, having a high mortality rate among the population. It often spreads to central nervous system and eye causing endophthalmitis, nonhepatic abscesses, pneumonia, necrotizing fasciitis, and meningitis. One visual trait of these strains is hypermucoviscous phenotype and a string test can be used to help the diagnosis.[21] Further examinations and treatments are made on a case-by-case basis, as there are currently no international guidelines.[22]
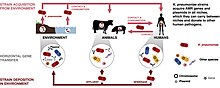
To get a K. pneumoniae infection, a person must be exposed to the bacteria. In other words, K. pneumoniae must enter the respiratory tract to cause pneumonia, or the blood to cause a bloodstream infection. In healthcare settings, K. pneumoniae bacteria can be spread through person-to-person contact (for example, contaminated hands of healthcare personnel, or other people via patient to patient) or, less commonly, by contamination of the environment; the role of transmission directly from the environment to patients is controversial and requires further investigation.[23] However, the bacteria are not spread through the air. Patients in healthcare settings also may be exposed to K. pneumoniae when they are on ventilators, or have intravenous catheters or wounds. These medical tools and conditions may allow K. pneumoniae to enter the body and cause infection.[24]
Klebsiella organisms are often resistant to multiple antibiotics. Current evidence implicates plasmids as the primary source of the resistance genes.[25] Klebsiella species with the ability to produce extended-spectrum beta-lactamases (ESBL) are resistant to virtually all beta-lactam antibiotics, except carbapenems. Other frequent resistance targets include aminoglycosides, fluoroquinolones, tetracyclines, chloramphenicol, and trimethoprim/sulfamethoxazole.[26]

Infection with carbapenem-resistant Enterobacteriaceae (CRE) or carbapenemase-producing Enterobacteriaceae is emerging as an important challenge in health-care settings.[28][29] One of many CREs is carbapenem-resistant Klebsiella pneumoniae (CRKP). Over the past 10 years, a progressive increase in CRKP has been seen worldwide; however, this new emerging nosocomial pathogen is probably best known for an outbreak in Israel that began around 2006 within the healthcare system there.[30] In the US, it was first described in North Carolina in 1996;[31] since then CRKP has been identified in 41 states;[32] and is routinely detected in certain hospitals in New York and New Jersey. It is now the most common CRE species encountered within the United States.
CRKP is resistant to almost all available antimicrobial agents, and infections with CRKP have caused high rates of morbidity and mortality, in particular among persons with prolonged hospitalization and those critically ill and exposed to invasive devices (e.g., ventilators or central venous catheters). The concern is that carbapenem is often used as a drug of last resort when battling resistant bacterial strains. New slight mutations could result in infections for which healthcare professionals can do very little, if anything, to treat patients with resistant organisms.
A number of mechanisms cause carbapenem resistance in the Enterobacteriaceae. These include hyperproduction of ampC beta-lactamase with an outer membrane porin mutation, CTX-M extended-spectrum beta-lactamase with a porin mutation or drug efflux, and carbapenemase production. The most important mechanism of resistance by CRKP is the production of a carbapenemase enzyme, blakpc. The gene that encodes the blakpc enzyme is carried on a mobile piece of genetic material (a transposon; the specific transposon involved is called Tn4401), which increases the risk for dissemination. CRE can be difficult to detect because some strains that harbor blakpc have minimum inhibitory concentrations that are elevated, but still within the susceptible range for carbapenems. Because these strains are susceptible to carbapenems, they are not identified as potential clinical or infection control risks using standard susceptibility testing guidelines. Patients with unrecognized CRKP colonization have been reservoirs for transmission during nosocomial outbreaks.[33]
The extent and prevalence of CRKP within the environment is currently unknown. The mortality rate is also unknown, but has been observed to be as high as 44%.[34] The Centers for Disease Control and Prevention released guidance for aggressive infection control to combat CRKP:
Israel 2007-2008. A nationwide outbreak of CRE in Israel peaked in March, 2007 at 55.5 cases per 100,000 patient days and necessitated a nationwide treatment plan. The intervention entailed physical separation of all CRE carriers and appointment of a task force to oversee efficacy of isolation by closely monitoring hospitals and intervening when necessary. After the treatment plan (measured in May, 2008), the number of cases per 100,000 patient days decreased to 11.7. The plan was effective because of strict hospital compliance, wherein each was required to keep detailed documentation of all CRE carriers. In fact, for each increase in compliance by 10%, incidence of cases per 100,000 patient days decreased by 0.6. Therefore, containment on a nationwide scale requires nationwide intervention.[36]
Nevada 2016. In mid-August 2016, a resident of Washoe County was hospitalized in Reno due to a CRE (specifically Klebsiella pneumoniae) infection. In early September of the same year, she developed septic shock and died. On testing by CDC an isolate from the patient was found to be resistant to all 26 antibiotics available in the US, including drug of last resort colistin.[37] It is believed she may have picked up the microbe while hospitalized in India for two years due to a broken right femur and subsequent femur and hip infections.[38][39][40]
Klebsiella pneumoniae carries a large number of anti-microbial resistance genes (AMR genes). These genes are transferred via plasmids from and to other human pathogens. One human pathogen that commonly acquires AMR genes from Klebsiella pneumoniae is Salmonella. This could help with treatment of salmonella infections due to having knowledge of possible antibiotic resistance data.
The majority of AMR genes in Klebsiella pneumoniae are plasmid-borne. An example of a niche would be soil, often considered a hotspot for gene transfer.
The table shows the number of AMR genes and plasmids (per strain or subspecies) compared to other common bacteria species.[41]
To prevent spreading Klebsiella infections between patients, healthcare personnel must follow specific infection-control precautions,[24] which may include strict adherence to hand hygiene (preferably using an alcohol based hand rub (60-90%) or soap and water if hands are visibly soiled. Alcohol based hand rubs are effective against these Gram-negative bacilli)[42] and wearing gowns and gloves when they enter rooms where patients with Klebsiella–related illnesses are housed. Healthcare facilities also must follow strict cleaning procedures to prevent the spread of Klebsiella.[24]
To prevent the spread of infections, patients also should clean their hands very often, including:
K. pneumoniae can be treated with antibiotics if the infections are not drug-resistant. Infections by K. pneumoniae can be difficult to treat because fewer antibiotics are effective against them. In such cases, a microbiology laboratory must run tests to determine which antibiotics will treat the infection.[24] More specific treatments of Klebsiella pneumonia are given in its section above. For urinary tract infections with multidrug-resistant Klebsiella species, a combination therapy with amikacin and meropenem has been suggested.[43]
Multiple drug-resistant K. pneumoniae strains have been killed in vivo by intraperitoneal, intravenous, or intranasal administration of phages in laboratory tests.[44] Resistance to phages is not likely to be as troublesome as to antibiotics as new infectious phages are likely to be available in environmental reservoirs. Phage therapy can be used in conjunction with antibiotics, to supplement their activity instead of replacing it altogether.[45]
New data sources outlining the global burden of K. pneumoniae and drug-resistant forms are expected to build momentum into prophylactic vaccine development.[46] The recent 2022 IHME study showed that in 2019 K. pneumoniae was responsible for 790,000 deaths [571,000 - 1,060,000] in all age groups across 11 infectious syndromes. Importantly, in Sub-saharan Africa K. pneumoniae was responsible for 124,000 [89,000-167,000] neonatal deaths due to bloodstream infections. Based on these and other data, a newly developed prophylactic vaccine would ideally be designed to prevent invasive K. pneumoniae disease in both vulnerable persons but also as a maternal vaccine to prevent neonatal sepsis and global demand assessments have been published.[47] As of June 2023, one single clinical development program for a K. pneumoniae vaccine [Kleb4V/GSK4429016A] was in a Phase 1/2 study in healthy adults aged 18-70 yrs (n=166) [Clinical trials identifier: NCT04959344]. The vaccine is an O-antigen based conjugate where the specific O-antigens in the vaccine remain undisclosed [Michael Kowarik, LimmaTech Biologics, World Vaccine Congress EU, 2022] although only a limited number of O-serotypes can account for a high proportion of clinical isolates. [48]
Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium. It appears as a mucoid lactose fermenter on MacConkey agar.
Although found in the normal flora of the mouth, skin, and intestines, it can cause destructive changes to human and animal lungs if aspirated, specifically to the alveoli resulting in bloody, brownish or yellow colored jelly-like sputum. In the clinical setting, it is the most significant member of the genus Klebsiella of the Enterobacteriaceae. K. oxytoca and K. rhinoscleromatis have also been demonstrated in human clinical specimens. In recent years, Klebsiella species have become important pathogens in nosocomial infections.
It naturally occurs in the soil, and about 30% of strains can fix nitrogen in anaerobic conditions. As a free-living diazotroph, its nitrogen-fixation system has been much-studied, and is of agricultural interest, as K. pneumoniae has been demonstrated to increase crop yields in agricultural conditions.
It is closely related to K. oxytoca from which it is distinguished by being indole-negative and by its ability to grow on melezitose but not 3-hydroxybutyrate.
Klebsiella pneumoniae es la especie de mayor relevancia clínica dentro del género bacteriano Klebsiella, compuesto por bacterias Gram negativas de la familia Enterobacteriaceae, que desempeñan un importante papel como causa de las enfermedades infecciosas oportunistas. El género fue llamado así en honor a Edwin Klebs, un microbiólogo alemán de finales del siglo XIX.
El bacilo ahora conocido como Klebsiella pneumoniae también fue descrito por Karl Friedländer, y durante muchos años se conoció como el «bacilo de Friedländer».
Son bacterias gram negativas, la asimilación y la fermentación de la lactosa se puede observar en el agar MacConkey donde las colonias son de color rosado y en el medio Kliger o TSI donde son Ácido/Ácido, es decir fermentador de la lactosa más producción de gas; y en la fermentación acetónica o prueba de Voges Proskauer son positivos. Por último, sus condiciones óptimas de cultivo son en agar nutritivo a 37 °C, pH de 7.0, presión osmótica de 1 atm.
Klebsiella pneumoniae, dentro de este género bacteriano, está implicada principalmente en infecciones nosocomiales.[1] Es el agente causal de infecciones del tracto urinario, neumonías, sepsis, infecciones de tejidos blandos, e infecciones de herida quirúrgica. Son especialmente susceptibles los pacientes ingresados en unidades de cuidados intensivos, neonatos, pacientes con EPOC, diabetes mellitus o alcohólicos.
Causa alrededor del 1% de las neumonías bacterianas y puede causar condensación hemorrágica extensa del pulmón. Además, en ocasiones provoca infección del aparato urinario y bacteriemia a partir de lesiones focales en pacientes debilitados que puede terminar con la vida del paciente. Algunas de las complicaciones más frecuentes son el absceso pulmonar y el empiema.
También suele encontrarse en las infecciones de la toracotomía para realización de by pass o revascularización coronaria. Suele responder en estos casos al imipenem, 1 g IV cada 6 horas por 21 días + ciprofloxacina, 400 mg IV cada 12 h por 21 días, acompañado todo esto de enérgica cura diaria realizada por el cirujano cardiovascular y colocación de Intrasite Gel (hidrogel de carboximetilcelulosa) cada 2 o 3 días dentro del lecho de la herida cuando ya no hay más secreción. El cierre por segunda intención es la regla.
El diagnóstico comienza a partir de la clínica; en los casos de neumonía, es especialmente útil el estudio radiográfico.
El diagnóstico definitivo lo obtenemos a partir del cultivo de muestras obtenidas de las mucosas del tracto respiratorio superior.
No se recomienda el uso de penicilinas, cefalosporinas (todas las generaciones), monobactames y carbapenemes independientemente de la sensibilidad in vitro. Opciones de tratamiento (requieren demostrar sensibilidad): agentes no beta-lactamicos.
Klebsiella pneumoniae es la especie de mayor relevancia clínica dentro del género bacteriano Klebsiella, compuesto por bacterias Gram negativas de la familia Enterobacteriaceae, que desempeñan un importante papel como causa de las enfermedades infecciosas oportunistas. El género fue llamado así en honor a Edwin Klebs, un microbiólogo alemán de finales del siglo XIX.
El bacilo ahora conocido como Klebsiella pneumoniae también fue descrito por Karl Friedländer, y durante muchos años se conoció como el «bacilo de Friedländer».
Son bacterias gram negativas, la asimilación y la fermentación de la lactosa se puede observar en el agar MacConkey donde las colonias son de color rosado y en el medio Kliger o TSI donde son Ácido/Ácido, es decir fermentador de la lactosa más producción de gas; y en la fermentación acetónica o prueba de Voges Proskauer son positivos. Por último, sus condiciones óptimas de cultivo son en agar nutritivo a 37 °C, pH de 7.0, presión osmótica de 1 atm.
Klebsiella pneumoniae Klebsiella generoko eta Enterobacteriaceae familiako espezie bat da, bakterio Gram negatiboz osatua dagoena. Klebsiellako espezien artean Klebsiella pneumoniae da patogenoa izan daitekeenetako bat (nahiz eta Klebsiella gehienak ez-kaltegarriak izan).
Bakterio hau ohikoa da gure heste-floran, eta bertan ez du inongo kalterik egiten. Baina inhalatzen denean pneumonia larri bat eragin dezake, batez ere osasun pattala dutenen artean, eta hortik datorkio izena.
Carl Friedländer mikrobiologo alemaniarrak XIX. mendearen bukaeran aurkitu zuen bakterio horrek zer ikusi handia zuela pertsona immunogutxituek duten pneumonia mota batekin. "Friedlanderen baziloa" deitu zitzaion urte askoz Klebsiella pneumoniaeri.
Espezie honen ezaugarri biokimiko nagusiak honako hauek dira:
Bakterio hau ez da mugikorra eta kapsula bat du horma zelularraren gainetik. Kapsula horrek bakterioaren birulentzia areagotzen du, leukozitoen fagozitosia eragozten duelako.
Bakterio hau ez da patogeno hertsia, oportunista baizik. Horrek esan nahi du immunitate-sistema ahula dutenak jotzen dituela gehienbat, hainbat patologia sortuz.
Pneumoniaz gain, gernu-aparatuko infekzioak ere sortzen ditu; ehun bigunen infekzioetan eta zauri infektatuetan ere parte har dezake Klebsiella pneumoniaek.
Infekzio nosokomial ugariren atzean dago. Klinika eta ospitaleetan immunogutxitu asko daude, oso sentikorrak direnak bakterio horren infekzioarekiko. Ez da makala inguru horietan bakterio horrek eragiten duen kaltea.
Hori gutxi balitz, antibiotiko askorekiko erresistentzia garatu du Klebsiella pneumoniaek, gaixoen tratamendua zailtzen duena.
Londresko Kings Collegen egindako ikerketek, bestalde, Klebsiella pneumoniaeren gainazaleko antigenoek oso antza handia zutela HLA-B27 giza antigeno leukozitarioarekin, eta mimetismo antigeniko horren ondorioz bakterio horrek eragin handia eduki lezakeela espondilitis ankilosatzailearen sorreran frogatu zuten.[1] [2]
Klebsiella pneumoniae Klebsiella generoko eta Enterobacteriaceae familiako espezie bat da, bakterio Gram negatiboz osatua dagoena. Klebsiellako espezien artean Klebsiella pneumoniae da patogenoa izan daitekeenetako bat (nahiz eta Klebsiella gehienak ez-kaltegarriak izan).
Bakterio hau ohikoa da gure heste-floran, eta bertan ez du inongo kalterik egiten. Baina inhalatzen denean pneumonia larri bat eragin dezake, batez ere osasun pattala dutenen artean, eta hortik datorkio izena.
Carl Friedländer mikrobiologo alemaniarrak XIX. mendearen bukaeran aurkitu zuen bakterio horrek zer ikusi handia zuela pertsona immunogutxituek duten pneumonia mota batekin. "Friedlanderen baziloa" deitu zitzaion urte askoz Klebsiella pneumoniaeri.
Espezie honen ezaugarri biokimiko nagusiak honako hauek dira:
anaerobio fakultatiboak: O/H: (+/+) oxidasa (-) katalasa (+) laktosaren hartzidura (+) glukosaren hartzidura (+), gasa sortuz IMVIC: (--++) Urearen hidrolisia: (+)Bakterio hau ez da mugikorra eta kapsula bat du horma zelularraren gainetik. Kapsula horrek bakterioaren birulentzia areagotzen du, leukozitoen fagozitosia eragozten duelako.
Klebsiella pneumoniae é unha especie bacteriana gramnegativa, inmóbil, encapsulada, fermentadora de lactosa, anaerobia facultativa, con forma bacilar. Aínda que se encontra na flora normal da boca, pel e intestinos,[1] pode causar problemas pulmonares se é aspirada. É unha enterobacteriácea e o membro clinicamente máis importante do xénero Klebsiella. Nos últimos anos, as klebsielas están converténdose en importantes patóxenos causantes de infeccións nosocomiais (hospitalarias).
Sete especies do xénero Klebsiella mostran grandes semellanzas na homoloxía do seu ADN. Estas son: Klebsiella pneumoniae, Klebsiella ozaenae, Klebsiella terrigena, Klebsiella rhinoscleromatis, Klebsiella oxytoca, Klebsiella planticola, e Klebsiella ornithinolytica. Ademais de K. pneumoniae, tamén se demostrou a presenza de K. oxytoca e K. rhinoscleromatis en mostras clínicas humanas.
K. pneumojniae está moi relacionada con K. oxytoca, da cal se pode distinguir por ser indol negativa e pola súa capacidade de crecer con melecitosa e beta-hidroxibutirato. Vive de forma natural no solo, e aproximadamente o 30% das cepas poden fixar o nitróxeno en condicións anaeróbicas.[2] Como diazótrofo de vida libre, o seu sistema de fixación do nitróxeno foi moi estudado.
Os membros do xénero Klebsiella expresan tipicamente dous tipos de antíxenos na súa superficie celular. O primeiro é o antíxeno O, que é un compoñente do lipopolisacárido (LPS), do cal existen 9 variedades. O segundo é o antíxeno K, un polisacárido capsular con máis de 80 variedades.[3] Ambos os dous contribúen á patoxenicidade e forman a base da distribución en serogrupos.
A fermentación de lactosa pódese observar en ágar MacConkey, onde forma colonias rosas, e no medio Kliger ou TSI, onde son fermentadoras de lactosa e produtoras de gas. Na probas de Voges Poskauer para a fermentación acetónica son positivas. Por último, as súas condicións óptimas de cultivo en ágar nutritivo son 37 °C e pH 7,0.
Klebsiella recibe o seu nome do bacteriólogo alemán Edwin Klebs (1834–1913). Tamén foi descrita por Karl Friedländer, e foi coñecido como "bacilo de Friedländer".
O científico danés Hans Christian Gram (1853–1938), desenvolveu a técnica da tinguidura de Gram en 1884 para distinguir entre K. pneumoniae e Streptococcus pneumoniae.
As infeccións por K. pneumoniae poden causar infeccións pulmonares (pneumonías). Causan cambios destrutivos nos pulmóns, con inflamación e hemorraxia, morte celular (necrose), e ás veces un esputo mucoide sanguento e mesto. O normal é que as bacterias cheguen ao pulmón cando a persoa aspira microbios que colonizaban a súa orofarinxe.
En xeral,, as infeccións por Klebsiella son máis frecuentes en persoas cun sistema inmunitario debilitado. Principalmente afectan a persoas de mediana idade ou vellas con enfermidades debilitantes, como diabetes, alcoholismo, tumores, enfermidades hepáticas, enfermidades pumonares obstrutivas crónicas, con terapia de glicocorticoides, insuficiencia renal, e certas profesións expostas (como a fabricación de papel). Moitas destas infeccións prodúcense en hospitais (enfermidades nosocomiais).
A infección máis común causada por Klebsiella adquirida fóra dos hospitais é a pneumonía por Klebsiella, tipicamente en forma de broncopneumonía e bronquite. Estes pacientes presentan un aumento da tendencia a desenvolver abscesos pulmonares, cavitación, empiema (pus na pleura), e adherencias. Presentan unha alta taxa de mortalidade de arredor do 50% mesmo se están recibindo terapia antibiótica. A taxa de mortalidade pode ser de case o 100% para as persoas con alcoholismo e bacteremia.
Ademais de pneumonía, Klebsiella pode tamén causar infeccións no tracto urinario, tracto biliar inferior, e feridas cirúrxicas, dando lugar a pneumonía, tromboflebite, infección do tracto urinario, colecistite, diarrea, infeccións do tracto respiratorio superior, infeccións en feridas, osteomielite, meninxite, e bacteremia e septicemia. Se unha persoa ten implantado un dispositivo invasivo no seu corpo o risco é maior; por exemplo os equipos de respiración asistida e catéteres urinarios aumentan o risco. Ademais, o uso de antibióticos pode ser un factor que incremente o risco de infección nosocomial por Klebsiella. A sepse e shock séptico poden orixinar a entrada da bacteria no sangue.
Investigacións realizadas no King's College de Londres atoparon que a imitación molecular do antíxeno HLA HLA-B27 por parte de dúas moléculas superficiais de Klebsiella estaba implicada na causa da espondilite anquilosante.[4]
Están aparecendo novas cepas de K. pneumoniae resistentes a antibióticos, que cada vez se detectan máis nas infeccións nosocomiais.[5]
A infección por enterobacteriáceas resistentes ao carbapenem (CRE) ou enterobacteriáceas produtoras de carbapenemase estase convertendo nun importante problema sanitario.[6] Entre elas está a Klebsiella pneumoniae resistente ao carbapenem (CRKP). Nos últimos 10 anos, estase producindo en todo o mundo un incremento progresivo destas cepas. En Israel houbo unha importante epidemia que empezou en 2006 e chegou ao máximo en marzo de 2007 con 55,5 casos por 100.000 pacientes e día, que obrigou a tomar importantes medidas sanitarias contra a epidemia en todo o país.[7] En EEUU o primeiro caso foi en 1996;[8] e despois foi detectada en 41 estados.[9]
A K. pneumoniae resistente ao carbapenem resiste a case todos os axentes microbianos dispoñibles, polo que ten unha alta taxa de morbilidade e mortalidade. O máis preocupante é que o carbapenem é xeralmente usado como antibiótico de último recurso contra as cepas bacterianas resistentes a outros varios antibióticos. Se se producisen novas mutacións que favorecesen a resistencia, as infeccións resultantes serían practicamene imposibles de tratar.
Hai varias formas en que as enterobacteriáceas adquiren resistencia ao carbapenem. Entre elas están: (1) Hiperprodución de ampC beta-lactamase cunha mutación na porina da membrana externa; (2) produción de beta-lactamase de espectro ampliado CTX-M cunha mutación na porina ou efluxo do fármaco, e (3) produción de carbapenemase. Cando K. pneumoniae produce o encima carbapenemase denomínase KPC ou K. pneumoniae resistente ao carbapenem (CRKP).[10]
Deles o mecanismo de resistencia máis importante é a produción do encima carbapenemase, blakpc. O xene que codifica o encima blakpc está situado nunha porción móbil do material xenético (un transposón; o transposón específico implicado denomínase Tn4401), o cal incrementa o risco de diseminación da bacteria. As CRE poden ser difíciles de detectar porque algunhas cepas que levan o blakpc teñen concentracións inhibitorias mínimas (concentración mínima á que o antimicrobiano inhibe o crecemento da bacteria) elevadas, pero que, malia seren elevadas, son aínda susceptibles ao rango de concentracións utilizadas cos carbapenems. Como esas cepas son susceptibles aos carbapenems, non son identificadas como riscos potenciais clínicos usando as directrices das probas de susceptibilidade estándar. Os pacientes con colonización por CRKP que non foron recoñecidas como tales funcionan como reservorios para a transmisión nosocomial.
A extensión e frecuencia dos CRKP no ambiente non se coñece actualmente. As infeccións por CRE nos hospitais deben tratarse con coidado tomando precaucións especiais cos pacientes infectados, illándoos, e comprobando os posibles casos doutros pacientes internados no hospital aos que non se lle detectaron anteriormente esas cepas.[11][12]
Unha esterilización efectiva e procedementos de descontaminación son medidas importantes para manter a taxa de infección por estas cepas resistentes o máis baixa posible.
Como ocorreu con moitas bacterias, o tratamento recomendado foi cambiando conforme o organismo desenvolveu resistencia ao mesmo. As Klebsiella son con frecuencia resistentes a múltiples antibióticos. As evidencias actuais indican que a fonte dos xenes de resistencia é un elemento xenético móbil. Klebsiella pola súa capacidade de producir beta-lactamases de espectro ampliado (ESBL) son resistentes a moitas clases de antibióticos. As resistencias máis frecuentes son a resistencia a aminoglicósidos, fluoroquinolonas, tetraciclinas, cloranfenicol, e trimetoprim/sulfametoxazol.[13]
A elección dun axente antimicrobiano específico depende da susceptibilidade das cepas do paciente e a parte do corpo que foi infectada. Para pacientes con infeccións graves, unha estratexia prudente é usar unha terapia combinada de antibióticos de curta duración (48-72 h), seguida dunha monoterapia específica unha vez que se coñece o patrón de susceptibilidade no paciente.
Se as Klebsiella específicas que presenta un paciente non presentan resistencia a antibióticos, entón os antibióticos utilizados son ampicilina/sulbactam, piperacilina/tazobactam, ticarcilina/clavulanato, ceftazidime, cefepime, levofloxacina, norfloxacina, gatifloxacina, moxifloxacina, meropenem, e ertapenem. Algúns expertos recomendan o uso de meropenem para pacientes con Klebsiella produtora de ESBL.
Ás veces é necesario a limpeza cirúrxica (por exemplo drenaxes) despois de iniciado o tratamento antibiótico.
Comprobouse que a K. pneumoniae morre in vivo pola administración de fagos por vía intraperitoneal, intravenosa ou intranasal, polo que se probou un tratamento con fagos.[14] Aínda que este tratamento estivo aplicándose durante un certo tempo, viuse que existe un maior risco de adquirir resistencia aos fagos que aos antibióticos. A resistencia aos fagos pode causar unha explosión no número destes microbios no ambiente e nos humanos (xa que non é un patóxeno obrigado e pode vivir fóra do corpo). Por iso a terapia de fagos úsase só en combinación con antibióticos.[15]
Klebsiella pneumoniae é unha especie bacteriana gramnegativa, inmóbil, encapsulada, fermentadora de lactosa, anaerobia facultativa, con forma bacilar. Aínda que se encontra na flora normal da boca, pel e intestinos, pode causar problemas pulmonares se é aspirada. É unha enterobacteriácea e o membro clinicamente máis importante do xénero Klebsiella. Nos últimos anos, as klebsielas están converténdose en importantes patóxenos causantes de infeccións nosocomiais (hospitalarias).
Sete especies do xénero Klebsiella mostran grandes semellanzas na homoloxía do seu ADN. Estas son: Klebsiella pneumoniae, Klebsiella ozaenae, Klebsiella terrigena, Klebsiella rhinoscleromatis, Klebsiella oxytoca, Klebsiella planticola, e Klebsiella ornithinolytica. Ademais de K. pneumoniae, tamén se demostrou a presenza de K. oxytoca e K. rhinoscleromatis en mostras clínicas humanas.
K. pneumojniae está moi relacionada con K. oxytoca, da cal se pode distinguir por ser indol negativa e pola súa capacidade de crecer con melecitosa e beta-hidroxibutirato. Vive de forma natural no solo, e aproximadamente o 30% das cepas poden fixar o nitróxeno en condicións anaeróbicas. Como diazótrofo de vida libre, o seu sistema de fixación do nitróxeno foi moi estudado.
Os membros do xénero Klebsiella expresan tipicamente dous tipos de antíxenos na súa superficie celular. O primeiro é o antíxeno O, que é un compoñente do lipopolisacárido (LPS), do cal existen 9 variedades. O segundo é o antíxeno K, un polisacárido capsular con máis de 80 variedades. Ambos os dous contribúen á patoxenicidade e forman a base da distribución en serogrupos.
A fermentación de lactosa pódese observar en ágar MacConkey, onde forma colonias rosas, e no medio Kliger ou TSI, onde son fermentadoras de lactosa e produtoras de gas. Na probas de Voges Poskauer para a fermentación acetónica son positivas. Por último, as súas condicións óptimas de cultivo en ágar nutritivo son 37 °C e pH 7,0.
Klebsiella pneumoniae è un batterio Gram-negativo, a forma di bastoncino, e, clinicamente, il membro più importante del genere Klebsiella (Enterobacteriaceae). Il nome del genere è un omaggio al batteriologo tedesco Edwin Klebs (1834–1913).
Klebsiella pneumoniae può provocare polmonite batterica, sebbene sia più comunemente coinvolta in infezioni acquisite in ospedale nel tratto urinario e in ferite, con particolare riferimento a individui immunocompromessi. Klebsiella pneumoniae è diventata una infezione nosocomiale in crescita dato che continuano ad apparire ceppi antibiotico-resistenti.
Lo scienziato danese Hans Christian Gram (1853–1928) ha sviluppato la tecnica nota come colorazione di Gram nel 1884 per distinguere la Klebsiella pneumoniae dallo Streptococcus pneumoniae.
Dalla Klebsiella pneumoniae viene estratta la klebsprotina (conosciuta anche con il nome di RU41740), una glicoproteina che in diversi studi sperimentali si è dimostrata efficace nel favorire la resistenza alle infezioni.
Di solito le persone sane non contraggono le infezioni da Klebsiella. Come l'Escherichia coli, i batteri del genere Klebsiella sono frequenti colonizzatori dell’intestino nell’uomo e in altri vertebrati dove non causano infezioni. Come regola generale, le infezioni da Klebsiella si osservano soprattutto nelle persone con un sistema immunitario indebolito. Spesso, l'infezione colpisce uomini di mezza età e anziani con malattie debilitanti: diabete, alcolismo, tumori maligni, malattie del fegato, malattie polmonari ostruttive croniche, insufficienza renale oppure soggetti sottoposti a terapia con glucocorticoidi. Per ottenere un'infezione da Klebsiella, organi e tessuti specifici devono essere esposti ai batteri. Ad esempio, la Klebsiella deve entrare nel tratto respiratorio per causare la polmonite, o nel sangue per causare un'infezione del sangue. Le persone che sono ricoverate in ospedale, curate per altre patologie, quando sono richiesti lunghi trattamenti con antibiotici o l'uso di ventilatori (respiratori) o cateteri sono più a rischio per le infezioni da Klebsiella. Le infezioni nosocomiali da Klebsiella sono causate principalmente da Klebsiella pneumoniae , la specie più importante dal punto di vista medico. In misura molto minore, è stata isolata la K. oxytoca. Si stima che la Klebsiella spp. causi l'8% di tutte le infezioni batteriche nosocomiali negli Stati Uniti e in Europa. Non sono state notate grandi variazioni geografiche nella frequenza. Negli Stati Uniti, la Klebsiella rappresenta dal 3 al 7% di tutte le infezioni batteriche nosocomiali, classificandole tra gli otto patogeni infettivi più importanti negli ospedali e dati raccolti dal Regno Unito e dalla Germania sono notevolmente simili.[1]Le infezioni localizzate più comuni sono nelle vie respiratorie e nel tratto urinario ma la Klebsiella è la seconda causa più comune di batteriemia da Gram-negativi. La gamma di malattie comprende anche tromboflebite, colecistite, diarrea, infezione del tratto respiratorio superiore, infezione delle ferite e dei siti chirurgici, osteomielite, meningite e setticemia.
I batteri Klebsiella si diffondono solitamente attraverso il contatto con le feci o con strumenti contaminati.[2][3][4]
I tassi di mortalità per la polmonite acquisita in ospedale di K. pneumoniae dipende sulla gravità della condizione sottostante, e può superare il 50% nei pazienti vulnerabili, anche se trattati con appropriati farmaci antibatterici.
In ambito nosocomiale si sono sviluppati anche ceppi di Klebsiella ipervirulenti e resistenti agli antibiotici.[5]
A partire dalla metà degli anni ‘80, è emersa una distinta sindrome delle infezioni invasive da K. pneumoniae acquisite in comunità, caratterizzata principalmente da ascessi epatici piogeni. Queste infezioni sono spesso complicate da devastanti infezioni metastatiche, tra cui endoftalmiti e meningiti. Contrariamente alla maggior parte delle altre infezioni da K. pneumoniae, circa la metà dei casi si verifica in giovani e individui altrimenti sani. Questa sindrome invasiva è stata per lo più segnalata in Asia: Taiwan, Corea del Sud, Cina, dove negli ultimi decenni la K. pneumoniae è diventata il più comune agente eziologico di ascesso epatico .
I ceppi di K. pneumoniae che causano queste infezioni invasive sono definiti ipervirulenti. Questa nuova variante è diversa dal ceppo classico in quanto l'aspetto delle colonie coltivate su una piastra di agar è ipermucoviscoso probabilmente per una sovraespressione dei polisaccaridi delle capsule.
La capsula è considerata come un importante fattore di virulenza nella K. pneumoniae in quanto proteggerebbe i batteri dalla fagocitosi e dall'azione battericida del siero.[6][7]
Similmente all'Escherichia coli, la K. pneumoniae può acquisire resistenza a più farmaci antibatterici principalmente attraverso il trasferimento orizzontale di elementi genetici mobili come i trasposoni o i plasmidi. La resistenza agli antibiotici spesso dipende da specifici enzimi in grado di idrolizzarli. I più diffusi e primi ad essere individuati sono stati i beta-lattamasi. La capacità di produrre beta-lattamasi a spettro esteso (in sigla ESBL) può rendere alcuni ceppi batterici resistenti a praticamente tutti gli antibiotici beta-lattamici, l’ampicillina e amoxicillina, cefalosporine ad ampio spettro ecc., ad eccezione dei carbapenemi.
In contrasto con l'Escherichia coli, ceppi della K. pneumoniae possono possedere uno specifico gene di resistenza (betalattamasi) localizzato cromosomicamente che rende naturalmente inefficaci gli antibiotici betalatamici.
Il fatto che i carbapenemi siano il trattamento di scelta per le infezioni gravi causate da ESBL, insieme ad una crescente incidenza di resistenza ai fluorochinoloni tra le Enterobacteriaceae, ha portato ad una maggiore dipendenza dai carbapenemi nella pratica clinica.[8][9][10][11]
Già nel 1994 in Giappone fu isolato un enzima metallo-beta-lattamasi di Classe B Ambler che come altri beta-lattamasi di classe A Ambler, isolati nel Nord America dal 2001, si dimostrò capace di idrolizzare gli antibiotici carbapenemi.[12][13][14][15][16]
L'infezione da Enterobacteriaceae resistenti ai carbapenemi (in sigla CRE) o da enterobatteriacee che producono carbapenemasi sta emergendo come una sfida importante nelle strutture sanitarie. La Klebsiella pneumoniae resistente ai carbapenemi (CRKP) è probabilmente il più noto CRE per un focolaio in Israele iniziato intorno al 2006 all'interno del sistema sanitario locale. Negli Stati Uniti la CRKP è la specie più diffusa di CRE.[17]
I batteri CRKP sono resistenti a quasi tutti gli agenti antimicrobici disponibili e le infezioni da CRKP hanno causato alti tassi di morbilità e mortalità, in particolare tra le persone con ospedalizzazione prolungata e quelle in condizioni critiche e esposte a dispositivi invasivi (ad esempio, ventilatori o cateteri venosi centrali). La preoccupazione è che i carbapenemi vengano spesso usati come farmaco di ultima istanza quando si combattono ceppi batterici resistenti. Nuove lievi mutazioni potrebbero provocare infezioni batteriche resistenti per le quali i professionisti sanitari possono fare molto poco per curare i pazienti.[18]
Alcuni studi[19] supportano l'esistenza di un mimetismo dei batteri Klebsiella pneumoniae con l'antigene di istocompatibilità umana HLA-B27. L'HLA-B27 regola importanti funzioni del sistema immunitario umano e la Klebsiella pneumoniae potrebbe scatenare reazioni autoimmuni. Una ricerca condotta al King's College di Londra ha indicato due molecole di superficie della Klebsiella come correlate alla spondilite anchilosante .[20]
Klebsiella pneumoniae è un batterio Gram-negativo, a forma di bastoncino, e, clinicamente, il membro più importante del genere Klebsiella (Enterobacteriaceae). Il nome del genere è un omaggio al batteriologo tedesco Edwin Klebs (1834–1913).
Klebsiella pneumoniae può provocare polmonite batterica, sebbene sia più comunemente coinvolta in infezioni acquisite in ospedale nel tratto urinario e in ferite, con particolare riferimento a individui immunocompromessi. Klebsiella pneumoniae è diventata una infezione nosocomiale in crescita dato che continuano ad apparire ceppi antibiotico-resistenti.
Lo scienziato danese Hans Christian Gram (1853–1928) ha sviluppato la tecnica nota come colorazione di Gram nel 1884 per distinguere la Klebsiella pneumoniae dallo Streptococcus pneumoniae.
Dalla Klebsiella pneumoniae viene estratta la klebsprotina (conosciuta anche con il nome di RU41740), una glicoproteina che in diversi studi sperimentali si è dimostrata efficace nel favorire la resistenza alle infezioni.
Klebsiella pneumoniae is een gramnegatieve staafvormige bacterie, die in de mond, darmen en op de huid voorkomt. Deze bacterie is een opportunist die met name in mensen met een verminderde weerstand infecties veroorzaakt. De bacterie is vernoemd naar de Duits-Zwitserse microbioloog Theodor Albrecht Edwin Klebs (1834-1913).
In de eerste maanden van 2007 overleden naar men vermoedt zo'n honderd ziekenhuispatiënten in Israël ten gevolge van deze bacterie.[1]
Van de Klebsiella pneumoniae is een multi-resistente variant ontstaan die als superbacterie nagenoeg onbehandelbare infecties geeft.
In juni 2011 werd door de overheid een onderzoek gedaan naar een mogelijke uitbraak van deze bacterie in het Maasstad Ziekenhuis te Rotterdam, dit naar aanleiding van 28 sterfgevallen in dat ziekenhuis. Uit onderzoek van het Maasstad Ziekenhuis, het RIVM en de GGD Rotterdam-Rijnmond is gebleken dat in totaal 98 patiënten de multiresistente KlebsiellaOxa-48 variant bij zich dragen, of in het verleden bij zich hebben gedragen.[2]
Eind mei werd de besmetting op de intensive care gemeld door het ziekenhuis, maar in februari ontdekte het Huisartsen Laboratorium in Etten-Leur al de resistente bacterie, althans volgens Omroep Brabant.
De bacterie zat in een urinemonster van een patiënt die in het Rotterdamse ziekenhuis was geopereerd.
Een screeningsonderzoek onder ruim 1800 ex-patiënten, waarmee het ziekenhuis in juli 2011 is begonnen, moest duidelijk maken hoeveel mensen drager van de bacterie zijn.
De Inspectie voor de Gezondheidszorg heeft eind juli 2011 het ziekenhuis onder verscherpt toezicht geplaatst omdat er onvoldoende vertrouwen was dat het ziekenhuis voldoende of de juiste maatregelen trof. Ook is rond diezelfde periode bij twee patiënten van het revalidatiecentrum Rijndam in Rotterdam de bacterie vastgesteld, een van de patiënten was eerder opgenomen in het Maasstad Ziekenhuis.
Na een onderzoek dat 29 maart 2012 is gepresenteerd is geconcludeerd dat er niet een specifieke 'dader' aanwijsbaar is voor het drama, maar dat er in het hele proces en op alle niveaus elementaire fouten zijn gemaakt.
In december 2015 is de bacterie aangetroffen bij zeker 16 patiënten die opgenomen waren in het Jeroen Bosch Ziekenhuis in Den Bosch.
Klebsiella pneumoniae is een gramnegatieve staafvormige bacterie, die in de mond, darmen en op de huid voorkomt. Deze bacterie is een opportunist die met name in mensen met een verminderde weerstand infecties veroorzaakt. De bacterie is vernoemd naar de Duits-Zwitserse microbioloog Theodor Albrecht Edwin Klebs (1834-1913).
In de eerste maanden van 2007 overleden naar men vermoedt zo'n honderd ziekenhuispatiënten in Israël ten gevolge van deze bacterie.
Van de Klebsiella pneumoniae is een multi-resistente variant ontstaan die als superbacterie nagenoeg onbehandelbare infecties geeft.
Klebsiella pneumoniae er ein gram-negativ bakterie som er vanleg i meltingssystemet hjå menneske.[1][2] Karbapenemasar, ei form for β-laktamasar, er kjende frå arten.[1]
I 2016 døydde ein pasient i Nevada i USA frå ein infeksjon av ei stamme av K. pneumoniae som var resistent mot alle 26 antibiotikum tilgjengelege for medisinsk bruk i USA. Pasienten vart truleg smitta nosokomielt gjennom sjukehusinnleggingar i India.[2]
Klebsiella pneumoniae er ein gram-negativ bakterie som er vanleg i meltingssystemet hjå menneske. Karbapenemasar, ei form for β-laktamasar, er kjende frå arten.
I 2016 døydde ein pasient i Nevada i USA frå ein infeksjon av ei stamme av K. pneumoniae som var resistent mot alle 26 antibiotikum tilgjengelege for medisinsk bruk i USA. Pasienten vart truleg smitta nosokomielt gjennom sjukehusinnleggingar i India.
Klebsiella pneumoniae er en gram-negativ stavformet bakterie. Bakterien er vanligvis en del av kroppens normalflora, men kan i visse tilfeller også forårsake alvorlig sykdom som lungebetennelse, særlig hos eldre og hos mennesker med redusert immunforsvar. Bakterien er i tillegg en hyppig årsak til såkalte sykehusinfeksjoner (nosokomiale infeksjoner). De senere årene har bakterien vært i særlig fokus på grunn av dens evne til å produsere såkalte ESBL, betalaktamaser med utvidet spektrum, og mange bakteriestammer er derfor svært antibiotikaresistente.[1]
Klebsiella pneumoniae er en gram-negativ stavformet bakterie. Bakterien er vanligvis en del av kroppens normalflora, men kan i visse tilfeller også forårsake alvorlig sykdom som lungebetennelse, særlig hos eldre og hos mennesker med redusert immunforsvar. Bakterien er i tillegg en hyppig årsak til såkalte sykehusinfeksjoner (nosokomiale infeksjoner). De senere årene har bakterien vært i særlig fokus på grunn av dens evne til å produsere såkalte ESBL, betalaktamaser med utvidet spektrum, og mange bakteriestammer er derfor svært antibiotikaresistente.
![]() Zapoznaj się z zastrzeżeniami dotyczącymi pojęć medycznych i pokrewnych w Wikipedii.
Zapoznaj się z zastrzeżeniami dotyczącymi pojęć medycznych i pokrewnych w Wikipedii.
Klebsiella pneumoniae é uma espécie de bactéria gram-negativa, encapsulada, anaeróbia facultativa em forma de bastonete. É o mais importante membro do género Klebsiella e importante membro da Família das enterobactérias.
Pode causar pneumonia, embora seja mais comum a sua relação com infecções hospitalares (aparelho urinário e feridas), em particular em doentes imunologicamente deprimidos, como portadores do vírus HIV/AIDS.
O nome Klebsiella advém do bacteriologista alemão Edwin Klebs.
Segundo um estudo chinês, a presença da Klebsiella pneumoniae no intestino pode ser responsável pela ocorrência de esteatose hepática (infiltração de gordura no fígado), devido ao seu metabolismo que transforma os carboidratos ingeridos em álcool.[1]
A Klebsiella pneumoniae está em crescimento em Portugal e noutros países da Europa provocando variados tipos de infecções graves com dificuldade de tratamento, pelo facto desta bactéria ser resistente a vários antibióticos, propagando-se com facilidade e provocando surtos.[2]
O aumento da infecção por esta bactéria deve-se “ao uso elevado de antibióticos, à livre circulação de pessoas que podem ser transportadoras dessas bactérias e também por haver alguma quebra das medidas básicas de controlo da infecção”.[2]
Para evitar a disseminação de infecções por Klebsiella entre pacientes, o pessoal de saúde deve seguir as precauções específicas de controle de infecção[3] que pode incluir estrita adesão à higiene das mãos (de preferência usando uma fricção à mão à base de álcool (60–90%) ou sabão e água se as mãos estiverem visivelmente sujas.[4] A vacina é outra opção.[5]
Klebsiella pneumoniae é uma espécie de bactéria gram-negativa, encapsulada, anaeróbia facultativa em forma de bastonete. É o mais importante membro do género Klebsiella e importante membro da Família das enterobactérias.
Pode causar pneumonia, embora seja mais comum a sua relação com infecções hospitalares (aparelho urinário e feridas), em particular em doentes imunologicamente deprimidos, como portadores do vírus HIV/AIDS.
O nome Klebsiella advém do bacteriologista alemão Edwin Klebs.
Klebsiella pneumoniae este o specie de bacterie Gram-negativă, imobilă, încapsulată, lactozo-fermentativă, facultativ anaerobă, care se prezintă sub formă de bacil.
Deși este răspândită în flora normală bucală, a pielii și intestinală,[1] Klebsiella pneumoniae poate să producă patologii oamenilor și animalelor, la nivelul plămânilor, inducând pneumonie cu eliminare de spută cu sânge sau mucus. Mai poate produce infecții de tract urinar sau meningite.[2] Este cel mai important reprezentat al genului Klebsiella din familia Enterobacteriaceae, iar speciile K. oxytoca și K. rhinoscleromatis au fost de asemenea întâlnite în cazuri clinice.
Klebsiella pneumoniae este o specie de bacterie Gram-negativă, imobilă, încapsulată, lactozo-fermentativă, facultativ anaerobă, care se prezintă sub formă de bacil.
Deși este răspândită în flora normală bucală, a pielii și intestinală, Klebsiella pneumoniae poate să producă patologii oamenilor și animalelor, la nivelul plămânilor, inducând pneumonie cu eliminare de spută cu sânge sau mucus. Mai poate produce infecții de tract urinar sau meningite. Este cel mai important reprezentat al genului Klebsiella din familia Enterobacteriaceae, iar speciile K. oxytoca și K. rhinoscleromatis au fost de asemenea întâlnite în cazuri clinice.
Bakterien Klebsiella pneumoniae förekommer ofta i tarmsystemet men kan i vissa fall ge upphov till olika former av infektioner hos människor, såsom urinvägsinfektion. Med undantag från resistensmekanismen Extended Spectrum Beta-Lactamase (ESBL) är inte förekomst av Klebsiella pneumoniae associerat med anmälningsplikt enligt smittskyddslagen.[1] K. pneumoniae kan även orsaka vårdrelaterade infektioner såsom lunginflammation, blodinfektioner, hjärnhinneinflammation samt sår eller infektioner i samband med kirurgiska ingrepp. Icke-resistenta infektioner kan behandlas med antibiotika.[2]
Bakterien Klebsiella pneumoniae förekommer ofta i tarmsystemet men kan i vissa fall ge upphov till olika former av infektioner hos människor, såsom urinvägsinfektion. Med undantag från resistensmekanismen Extended Spectrum Beta-Lactamase (ESBL) är inte förekomst av Klebsiella pneumoniae associerat med anmälningsplikt enligt smittskyddslagen. K. pneumoniae kan även orsaka vårdrelaterade infektioner såsom lunginflammation, blodinfektioner, hjärnhinneinflammation samt sår eller infektioner i samband med kirurgiska ingrepp. Icke-resistenta infektioner kan behandlas med antibiotika.
Klebsiella pneumoniae Gram negatif, kendiliğinden hareketi olmayan, kapsül içerisinde, fermantasyon yapabilen bir bakteri türüdür.
Normal flora olarak, ağız, deri ve bağırsakta olmasına rağmen, aspire edilmesi durumunda, akciğerde yıkıcı hasarlara neden olmaktadır.<[1] Klinik olarak incelendiğinde Klebsiella sınıfının Enterobacteriaceae şubesinin bir üyesidir. Ayrıca K oxytoca ve K rhinoscleromatis bakterileride insanda enfeksyon yapan bakteri türleri arasında gösterilebilir. Son yıllarda K. Pneumoniae nazokomiyal enfeksyonlarda önemli bir patojen olmuştur.
K. Pneumonia normal olarak toprakta bulunur ve soylarının 30%'u azot fiksasyonu ile aneorob olarak yaşamlarını sürdürürler.[2] Diazotrof olarak serbest olarak yaşayan, K. Pneumoniae azot fiksasyonu için çok önemli olduğu, ve tarım ürünlerinin verimliğinde çok önemli rol üstlendiği görülmüştür.[3]
Klebsiella türünün üylereinde, hücre yüzeyinde iki tip antijen bulunur. İlk olanı, 9 çeşitte bulunan liposakkarit yapıdaki O antijenlerdir. İkincil olarak K antijeni 80 küsur çeşidi bulunan kapsüllü polisakkarit şeklinde olanlardır. Her iki serotip için de patojenik etkisi vardır.[4]
K. Pneumoniae, indole-negative testiyle ayrılan, ve her iki metazitos ve 3-hydroxybutyrate ile büyüme kabiliyeti olan K. Oxycota' ile akrabalığı vardır.
Danimarkalı bilim adamı Hans Christian Gram (1853–1938), Gram boyama olarak bilinen tekniği geliştirmiş ve 1884'te K. Pneumoniea'dan Streptococcus pneumoniae'yı ayırt etmiştir.
Klebsiella türü daha sonra Alman bakteriyolojist Edward Klebs (1834–1913) tarafından ismlenirilmiştir.
K.pneumoniae, yoğun prülan balgam üreten, enflamasyon, hücre ölümleri ve hemoroji ile akciğerlerde kalıcı hasarlara sebep olabilir. Bu tipteki bakteriler orofaringeal mikroorganizma kolonilerinin alt solunum yolu extremitelerine aspire edilmesi ile kazanılır.
Genel kural olarak, Klebsiella düşük immün sistemli kişilerde daha çok enfeksyona yol açar. Hastalık genellikle direnci zayıf orta yaşlı veya yaşlı kişileri etkiler. Hasta popülasyonu genellikle, respritüel konak savunması azalan, diyabet, alkolizm, kanser, karaciğer hastalığı, KOAH, glükokortikoidterapisi ve kronik böbrek yetmezliği olan hastalarda bulaşma oranı yüksektir. Bu bakteri hastanede yatan bir hastadan diğer hastaya geçebilmektedir. Feçes en önemli bulaşma yoludur ve kontamine eşyaların kullanımı da enfeksyon bulaşma riskini artırmaktadır.
Hastane dışında Klebsiella bakterisinin neden olduğu en yaygın enfeksyon pnömoni'dir. En tipik şekli ise bronkopnömoni ve bronşittir. Bu hastalar, akciğer absesi, ampiyemi, kavitasyon ve üral adhezyon gelişmesine meylederler. Antimikrobiyal tedaviye karşın ölüm oranı %50'dir. Mortalite oranı en yüksek olanlar hastalar alkolizm ve bakteriyemi ile mücadele eden hastalardır.
Pnömoniye ek olarak, Klebsiella Üriner sistemde, safra kanalında ve cerrahi girişim yapılan yerde enfeksyona sebep olabilir. Klinik hastalık aralığı olarak, tromboflebit, idrar yolu enfeksiyonu (İYE), kolesistit, ishal, üst solunum yolu enfeksiyonu, yara enfeksiyonu, osteomiyelit, menenjit, bakteriyemi ve septisemiyi içermektedir. İnvaziv bir cihaz veya komponent olan hastalrda, cihazın kontamine olma riski yüksektir; solunum destek ekipmanları ve üriner katater risk faktörü yüksek olan hastalar kategorisine koyar. Antibiyotik kullanımı da nazokomiyal olarak Klebsiella'nın etkinliğini artırır. Bakterinin kana bulaşması sepsis veya septik şok gelişir.
Klebsiella'/nowiki>nın rinoskleroma ve kronik atrofik rinit adında iki tana olağandışı hastalığı mevcuttur. Rinoskleroma nazofarenksin kronik enflamasyon sürecinde rol oynayan bir hastalıktır. Kronik atrofik rinit ise nazal mukoza'nın nekroze uğramasını ifade eder.
Klebsiella yaşlı kişilerde idrar yolu enfeksyonu için E.Coli'den sonra ikinci sırada yer almaktadır. Ayrıca, kronik akciğer hastalığı, enterik patojenik, burun mukozası atrofisi ve rinoskleroma olan hastalar için bir fırsatçı patojendir.
K. pneumoniae'nın eni antibiyotik karşı dirençli suşlar ortaya çıkmaktadır.
Klebsiella organizması çoğu antibiyotiğe karşı dirençlidir. Mevcut kanıtlar, dirençli genlerin kaynağı olarak, bir plazmitin etkisi olduğunu göstermektedir. Geniş spektrumlu beta-laktamaz GSBL üretme yeteneği olan Klebsiella çoğu antibiyotiğe direnç göstermektedir. Çoğunlukla direnç gösterdikleri aminoglikozitler, florokinolonlar, tetrasiklinler, kloramfenikol ve trimetoprim-sulfametoksazoldür.
Karbapeneme dirençli Enterobacteriaceae (CRE) veya karbapenemaz üreten Enterobacteriaceae ile enflamasyon sağlık alanında önemli bir sorun olarak ortaya çıkmaktadır. Karbapenem dirençli Enterobacteriaceae'lerin birçoğu (CRE) bir karbapenem dirençli Klebsiella pneumoniae (CRKP) olduğundur. Geçen on yıl içersinde, dünya çapında CRKP'nin sürekli bir artış eğiliminde olduğu görülmüştür; Ancak bu yeni çıkan nazokomiyal enfeksiyon, 2006 yılında İsrail'de en iyi sağlık koşulları olarak bilinen bir dönemde salgın oluşturmuştur. İlk olarak ABD'de Kuzey Caroline'de tanımlanmıştır. O zamandan beri CRKP 41 eyalette tespit edilmiştir; New York ve New Jersey gibi bazı hastanelerde rutin olarak kazanılmıştır.[5] Şimdi ise Amerika Birleşik Devletleri içerisinde en sık karşılaşılan CRE türdür.
CRKP neredeyse tüm antimikrobiyal ajanlara dirençlidir ve CRKP ile enfekte olmuş hastaların uzun süreli yatşları, invaziv girilşimlere ve cihazlara (örneğin; ventlatörler, santral venöz kataterler) maruz kalan hastalarda morbidite ve mortalite oranının yüksek olmasına neden olmuştur. Karbapanem dirençli suşları ile mücadelede son çare olarak kullanılan ilaçlardandır. Kücük yeni mutasyonlar eğer enfeksyona sebep oluyorsa, sağlık çalışanılarının bu dirence karşı yapabilecekleri çok az şey vardır.
|coauthors= görmezden gelindi (yardım) Klebsiella pneumoniae Gram negatif, kendiliğinden hareketi olmayan, kapsül içerisinde, fermantasyon yapabilen bir bakteri türüdür.
Normal flora olarak, ağız, deri ve bağırsakta olmasına rağmen, aspire edilmesi durumunda, akciğerde yıkıcı hasarlara neden olmaktadır.
K. pneumoniae — грам-негативна, дрібна (0,5-0,8 × 1-2 мікрон) паличкоподібна бактерія. Не утворює спор, нерухома. Здібна до утворення капсули. Розташовуються поодинці, попарно і скупченнями. Легко забарвлюються аніліновими фарбниками.
K. pneumoniae — хемоорганогетеротроф, факультативний анаероб. Культивується на простих живильних середовищах (МПА, МПБ). На агаризованих живильних середовищах утворює круглі слизисті сірувато-білі колонії. У МПБ — рівномірне помутніння середовища з утворенням тягучого слизистого осаду і плівки. Зброджує лактозу, не утворює індолу.
K. pneumoniae є одним зі збудників пневмонії[2], а також інфекцій сечостатевої системи[3], гнойних абсцесів печінки[4][5], селезінки[6][7][8]. Викликає гнойні та фіброзні плеврити, перикардити, гайморити, ендофтальмити. Важливий збудник нозокоміальних інфекцій. Ці бактерії також патогенні для деяких тварин[9]. Деякі штами бактерії мають полірезистентність до багатьох антибіотиків, обумовлену наявністю R-плазміди, також у деяких штамів проявляється стійкість до карбапенемів за рахунок наявності карбапенем-гідролізуючих β-лактамаз[10][11]. Капсула, що формується цими бактеріями деяких штамів, також є фактором патогенності[12].
|voluem= (довідка) |voluem= (довідка) |voluem= (довідка)
Klebsiella pneumoniae
(Schroeter 1886) Trevisan 1887
Klebsiella pneumoniae (лат., клебсиелла пневмонии, палочка Фридлендера) — вид грамотрицательных факультативно-анаэробных палочковидных бактерий. Выделена в 1882 году немецким микробиологом Карлом Фридлендером.
Один из возбудителей пневмонии, также ассоциирована с инфекциями мочеполовой системы[2], внутрибольничными инфекциями.
Грамотрицательная, мелкая (0,5—0,8 × 1—2 мкм) коккобацилла. Спор не образует, неподвижна. Способна к образованию капсулы. Располагаются одиночно, попарно и скоплениями. Легко окрашиваются анилиновыми красителями.
Хемоорганогетеротроф, факультативный анаэроб. Культивируется на простых питательных средах (МПА, МПБ). На агаризованных питательных средах образует круглые слизистые серовато-белые колонии. В МПБ — равномерное помутнение среды с образованием тягучего слизистого осадка и плёнки. Сбраживает лактозу, не образует индола.
K. pneumoniae является одним из возбудителей пневмонии[3], а также урогенитальных инфекций[4], гнойных абсцессов печени[5][6], селезёнки[7][8][9][10]. Вызывает гнойные и фиброзные плевриты, перикардиты, гаймориты, эндофтальмиты. Важный возбудитель нозокомиальных инфекций. Микроорганизм также патогенен и для некоторых животных[11]. Некоторые штаммы обладают полирезистентностью к антибиотикам, обусловленную наличием R-плазмиды[12], также проявляется устойчивость к карбапенемам за счёт наличия карбапенем-гидролизующих β-лактамаз[13][14]. Капсула является фактором вирулентности[15].
Klebsiella pneumoniae (лат., клебсиелла пневмонии, палочка Фридлендера) — вид грамотрицательных факультативно-анаэробных палочковидных бактерий. Выделена в 1882 году немецким микробиологом Карлом Фридлендером.
Один из возбудителей пневмонии, также ассоциирована с инфекциями мочеполовой системы, внутрибольничными инфекциями.
克雷伯氏肺炎菌Klebsiella pneumoniae
克雷伯氏肺炎菌(Klebsiella pneumoniae)屬於革蘭氏陰性菌,桿狀,有大量黏性的多醣形成的莢膜包覆。克雷伯氏肺炎菌可以在人類,特別是免疫力低弱的個體造成肺炎、尿路感染、菌血症等等感染症。[1][2]然而除了院內感染之外,近年來社區型感染也漸漸增加。在台灣的糖尿病病患中,克雷伯氏菌經常是造成肝膿瘍(liver abscess)的病源。[3] 克雷伯氏菌可以進行乳糖發酵,屬於兼性厭氧生物,它是目前檸檬酸發酵被研究的最清楚的微生物[4]。
西元1884年丹麥科學家漢斯·克里斯蒂安·革蘭(1853年-1938年)研發了革蘭氏染色法,當初即是用來分辨肺炎鏈球菌(屬革蘭氏陽性)與克雷伯氏肺炎菌(革蘭氏陰性)。
德國微生物學家艾德溫·克雷伯(Edwin Klebs)首先描述了克雷伯氏肺炎菌,此菌後來因此得名。
1996年,美國北卡羅納州出現具有A類碳青黴烯酶的克雷伯氏肺炎菌,全名為「克雷伯肺炎桿菌碳青黴烯酶」(KPC,Klebsiella pneumoniae carbapenemase),能使碳青黴烯類抗生素失去藥效,致死率30%~60%[5]。
克雷伯氏肺炎菌(Klebsiella pneumoniae)屬於革蘭氏陰性菌,桿狀,有大量黏性的多醣形成的莢膜包覆。克雷伯氏肺炎菌可以在人類,特別是免疫力低弱的個體造成肺炎、尿路感染、菌血症等等感染症。然而除了院內感染之外,近年來社區型感染也漸漸增加。在台灣的糖尿病病患中,克雷伯氏菌經常是造成肝膿瘍(liver abscess)的病源。 克雷伯氏菌可以進行乳糖發酵,屬於兼性厭氧生物,它是目前檸檬酸發酵被研究的最清楚的微生物。

クレブシエラ・ニューモニエ(Klebsiella pneumoniae)とは、グラム陰性の桿菌で、日本では肺炎桿菌とも呼ばれる。口腔や腸管における常在菌であるが、しばしば呼吸器感染症、尿路感染症などを引き起こす。弱毒菌であるが、菌交代現象を起こし、感染症を引き起こし問題となる。
学名は属名がドイツの細菌学者Edwin Klebsへの献名で、種形容語がギリシア語で肺炎を意味するΠνευμονία(Pneumonia)に由来する。ラテン語風にクレブシエラ・プネウモニアエとも呼ぶ。
クレブシエラ属の基準種に指定されている。
大葉性肺炎の病態を取ることが多く、また胸部X線写真上では緊満性病変による葉間胸膜圧迫像(bulging sign)を形成することがある。治療には、第二・三世代セフェム系抗生物質やニューキノロン系抗菌剤が用いられる。
폐렴막대균 또는 폐렴간균(학명: Klebsiella pneumoniae, 클렙시엘라 뉴모니아)은 그람 음성, 비운동성, 락토스 발효, 통성 혐기성, 막대 모양의 협막을 가지는 세균이다. 맥콘키 선택배지에서 점액성(mucoid)의 락토스 발효 세균으로 나타난다.
입, 피부, 장의 정상세균총에서 발견되지만[1], 흡인하면 인간과 동물의 폐, 특히 폐포에 파괴적인 변화를 일으켜 가래와 같은 피 묻은 갈색, 또는 노란색의 젤리 같은 점액을 만들 수 있다. 임상 환경에서 폐렴막대균은 장내세균과에 속한 클렙시엘라속의 가장 중요한 세균이다. 클렙시엘라속에 속한 다른 세균인 K. oxytoca 나 K. rhinoscleromatis는 인간 임상 표본에서도 존재가 입증되었다. 최근 몇 년 동안 클렙시엘라속에 속한 종들은 병원 감염에서 중요한 병원체로 떠올랐다.
토양에서 자연적으로 발생하며 약 30%의 균주가 혐기성 조건에서 질소 고정을 일으킬 수 있다.[2] 이 때문에 자유 생활 질소 고정 세균으로서 질소 고정 시스템이 많이 연구되었다. 폐렴막대균이 농업에서 작물 수확량을 증가시키는 것으로 입증되었기 때문에, 농업적인 흥미의 대상이 된다.[3]
클렙시엘라 옥시토카와 밀접한 관계에 있다. 폐렴막대균은 인돌 검사 음성이며 3-하이드록시부티레이트가 아닌 멜레치토스에서 자라는 능력으로 구별된다.
클렙시엘라속의 이름은 독일의 미생물학자 에드윈 클렙스(Edwin Klebs, 1834-1913)의 이름을 따서 명명되었다. 폐렴막대균은 독일의 병리학자 카를 프리드랜더(Carl Friedländer)를 기리기 위해 Friedlander's bacillum으로도 알려져 있는데, 그는 이 세균이 특히 만성 질환자나 알코올 중독자 등 면역 저하자에게서 나타나는 폐렴의 원인이라고 제안했다.[4]
폐렴막대균의 감염은 다른 쇠약해지는 질병이 있는 중년, 노년층에게 주로 영향을 미친다. 이 환자 집단은 당뇨병, 알코올 의존증, 암, 간 질환, 만성 폐쇄성 폐질환, 글루코코르티코이드 요법, 신부전, 특정 직업(예: 제지 공장 작업자) 등 호흡기 방어가 손상된 환자들로 여겨진다. 이러한 환자들에서 폐렴막대균 감염의 대부분은 환자가 다른 이유로 병원에 입원할 때 일어나는, 원내감염의 형태로 이루어진다.
폐렴 외에도 클렙시엘라는 비뇨계통, 하부 담도, 수술 상처 부위에 감염을 일으킬 수 있다. 임상 질환에는 폐렴, 혈전정맥염, 요로감염증, 쓸개염, 설사, 상기도감염증, 상처 감염, 골수염, 수막염, 균혈증, 패혈증 등이 있다. 신체에 침습적 장치가 있는 환자의 경우 장치가 폐렴막대균에 오염될 위험이 있다. 신생아 병동의 장치, 호흡을 돕기 위한 장비, 요도 카테터는 환자를 위험에 빠뜨릴 수 있다. 또한, 항생제 사용은 클렙시엘라에 의한 원내 감염의 위험을 증가시키는 요인이 될 수 있다. 패혈증과 패혈성 쇼크는 세균이 혈액으로 들어가면 발생할 수 있다.
킹스 칼리지 런던에서 수행된 연구에 따르면 HLA-B27과 클렙시엘라 세균의 두 표면 분자 사이의 분자 모방이 강직성 척추염의 원인과 관련되어 있다.[5]
클렙시엘라는 노인의 요로감염증을 대장균 다음으로 많이 일으킨다.[6] 또한 만성 폐질환, 장 질환, 코점막의 위축, 코경화증이 있는 환자에게 기회감염 병원체가 될 수 있다. 새로운 항생제 내성을 가진 폐렴막대균 균주도 나타나고 있다.[7]
병원 외부의 클렙시엘라 세균에 의해 유발되는 가장 흔한 상태는 일반적으로 기관지폐렴 형태로 나타난 폐렴이나 기관지염이다. 발병한 환자에서는 폐 농양, 공동화, 농흉, 가슴막 유착이 발생하는 경향이 크다. 항생제 치료를 시행해도 사망률이 약 50%이다.
이런 형태의 폐렴은 일반적으로 흡인으로 인해 발생하며 알코올 의존증이 위험 인자일 수 있다. 또한 일반적으로 원내 요로 감염이나 COPD 환자와 관련이 있다.[8][9]
폐렴막대균의 병태생리학 측면에서 주목할 것은 폐렴막대균에 대한 호중구의 골수세포형과산화효소를 통한 방어이다. 엘라스테이스의 산화적 비활성화가 폐렴막대균 방어에 관여하며, 지질다당류 결합 단백질(LBP)은 세균의 세포벽 구성 요소를 세포로 전달하는 데 도움이 된다.[10][11]
클렙시엘라 폐렴이 있는 사람은 특징적인 가래를 기침으로 내뱉으며, 발열, 메스꺼움, 빈맥, 구토 증상을 보이는 경향이 있다. 클렙시엘라 폐렴은 알코올 중독과 같은 기저질환이 있는 사람들에게 영향을 더 많이 미친다.[8]
클렙시엘라 폐렴 진단과 관련하여 폐렴막대균에 환자가 감염되었는지 판별하기 위해 광범위 베타 락타메이스(Extended Spectrum β-Lactamase, ESBL)에 대한 감수성 검사나 다음의 검사들을 수행할 수 있다.[10][8]
클렙시엘라 폐렴의 치료는 아미노글리코사이드나 세팔로스포린과 같은 항생제에 의해 이루어지며, 개인의 건강 상태, 병력, 질병의 중증도에 따라 사용할 항생제를 선택한다.[9][12]
클렙시엘라는 암피실린에 대한 내성을 가지도록 만드는 베타-락타메이스를 보유하고 있으며, 많은 균주가 카르베니실린, 아목시실린, 세프타지딤에 대한 추가 내성과 함께 광범위 베타-락타메이스를 획득했다. 폐렴막대균은 아미노글리코사이드와 세팔로스포린에 대해서는 감수성을 보이며, 베타-락타메이스 억제제인 클라불란산으로 베타-락타메이스를 얼마나 억제 가능한지는 다양한 것으로 보고되었다. 집중치료실(ICU)에서 다제내성 그람 음성 병원균으로 인한 감염은 콜리스틴이 다시 사용되게 만들기도 하였다. 그러나 폐렴막대균의 콜리스틴 내성 균주가 ICU에서 보고되었다.[10][13][14][15] 2009년 인도와 파키스탄에서 정맥 주사용 항생제인 카바페넴에 내성을 보이는 뉴델리 메탈로-베타락타메이스(NDM-1)라는 유전자를 가진 폐렴막대균이 발견되었다. 대만의 클렙시엘라 발병 사례는 당뇨병(DM)이 있는 사람들에게 간농양을 유발하는 비정상적인 독성을 나타냈으며, 치료는 3세대 세팔로스포린으로 이루어졌다.
고병원성 폐렴막대균(hypervirulent Klebsiella pneumoniae, hvKP)은 아시아에서 발생한 높은 사망률의 매우 치명적인 변종이다. 종종 중추신경계와 눈으로 퍼지고, 눈에서는 안구내염을 유발한다. 현재 국제적인 지침은 없기 때문에 추가 검사와 치료는 사례별로 다르게 이루어진다.[16]
폐렴막대균 감염에 걸리려면 우선 사람이 세균에 노출되어야 한다. 즉, 폐렴막대균이 기도로 들어가야 폐렴을 일으킬 수 있으며, 또는 혈액으로 들어가 혈류를 통한 감염을 일으킬 수도 있다. 의료 환경에서 폐렴막대균은 사람 대 사람 접촉(예: 의료진의 오염된 손을 통해서 전달, 환자 간에 전달)을 통해 전파되거나, 덜 흔한 방법이지만 환경 오염에 의해서도 전파될 수 있다. 환경에서 환자로 직접 전파되는 방식에는 논란의 여지가 있으며 추가 조사가 필요하다.[17] 그러나 공기를 통해서는 전파되지 않는다. 의료 환경에 있는 환자는 인공호흡기나 정맥 카테터를 사용하는 중인 경우, 또는 상처가 있는 경우에도 폐렴막대균에 노출될 수 있다. 이러한 의료 도구와 조건으로 인해 폐렴막대균이 몸에 들어가 감염을 일으킬 수 있다.[18]
클렙시엘라에 속하는 세균들은 종종 여러 항생제에 내성이 있다. 현재까지 나온 연구를 통한 근거들은 플라스미드가 저항성 유전자의 기원이라고 보고 있다.[19] 광범위 베타-락타메이스(ESBL)를 생산하는 능력을 가진 클렙시엘라 종은 카바페넴을 제외한 거의 모든 베타-락탐 항생제에 내성이 있다. 빈번하게 내성을 가지는 다른 항생제에는 아미노글리코사이드, 플루오로퀴놀론, 테트라사이클린, 클로람페니콜, 트리메토프림/설파메톡사졸이 있다.[20]
카바페넴 내성 장내세균(CRE), 또는 카바페네메이스를 생산하는 장내세균과 감염은 의료 환경에서 중요한 도전 과제로 떠오르고 있다.[22][23] 많은 CRE 중 하나가 카바페넴 내성 폐렴막대균(carbapenem-resistant Klebsiella pneumoniae, CRKP)이다. 지난 10년 동안 전 세계적으로 CRKP는 점진적으로 증가해 왔다. 그러나 이 새로운 원내감염 병원체는 2006년경 이스라엘의 의료 시스템 내에서 시작된 발병으로 가장 잘 알려져 있다.[24] 미국에서는 1996년 노스캐롤라이나에서 처음 기술되었다.[25] 그 이후로 CRCP는 미국의 41개 주에서 확인되었다.[26] 뉴욕과 뉴저지의 특정 병원에서 일상적으로 발견된다. 현재 미국 내에서 가장 흔한 CRE 종이다.
CRKP는 사용 가능한 거의 모든 항균제에 내성이 있다. 특히 장기간 입원한 사람과 중환자, 침습적 장치(예: 인공호흡기나 중심 정맥 카테터)에 노출된 사람에게 잘 감염되어, 높은 이환율과 사망률을 보였다. 우려되는 점은 카바페넴이 항생제 내성 균주와 싸울 때 최후의 수단으로 사용되는 경우가 많다는 점이다. 새로운 약간의 돌연변이로 인해 의료 전문가가 내성 병원체에 감염된 환자를 치료하기 위해 거의 아무것도 할 수 없는 감염이 발생할 수 있다.
많은 기전이 장내세균과에서 카바페넴 내성을 유발한다. 여기에는 외막 포린 돌연변이가 있는 ampC 베타-락타메이스의 과다 생산, 포린 돌연변이가 있거나 약물을 유출시키는 CTX-M 광범위 베타-락타메이스, 카바페네메이스 생산 등이 있다. CRKP에 의한 내성의 가장 중요한 기전은 카바페네메이스 효소인 blakpc의 생산이다. blakpc 효소를 암호화하는 유전자는 유전 물질(트랜스포존, 관련된 특정 트랜스포존을 Tn4401이라고 함)의 이동 가능한 조각을 통해 운반되어 전파 위험을 높인다. blak pc를 보유하는 일부 균주는 증가된 최소 억제 농도를 갖지만, 여전히 카바페넴에 대한 감수성 범위 내에 있기 때문에 CRE라는 것을 감지하기 어려울 수 있다. 이러한 균주는 카바페넴에 여전히 민감하기 때문에 표준 감수성 테스트 지침을 사용하였을 때는 감염 통제의 위험으로 식별되지 않는다. 이런 식으로 인식되지 못한 CRKP 집락이 있는 환자는 병원 발병 동안 전염을 위한 저장소 역할을 하게 되었다.[27]
환경 내에서 CRCP의 범위와 유병률은 현재 알려져 있지 않다. 사망률도 알려져 있지 않지만 44% 정도까지 높은 것으로 관찰되었다.[28] 미국 질병통제예방센터(Centers for Disease Control and Prevention)는 CRCP 퇴치를 위한 적극적인 감염 통제 지침을 발표했다.
이 봉쇄 정책의 한 가지 구체적인 예는 2007년 이스라엘에서 볼 수 있다.[30] 이 정책이 시행됐던 기간은 2007년 4월부터 2008년 5월까지였다. 당시 이스라엘에서는 CRE의 전국적 발병(2007년 3월 환자 100,000일당 발병 55.5건으로 정점을 찍음)으로 전국적인 치료 계획이 필요했다. 개입에는 모든 CRE 보균자를 물리적으로 분리하고, 병원을 면밀히 모니터링하고, 필요할 때 개입함으로써 격리의 효율성을 감독하기 위한 태스크 포스의 임명이 이루어졌다. 치료계획(2008년 5월 측정) 이후 환자 10만 명당 발병 건수는 11.7건으로 감소했다. 이 계획은 엄격한 병원 규정 준수로 인해 효과적이었다. 각 병원에서는 모든 CRE 보균자에 대한 자세한 문서를 보관해야 했다. 실제로, 순응도가 10% 증가할 때마다 환자 10만 명당 사례 발생률은 0.6씩 감소했다. 따라서 전국적인 규모의 봉쇄에는 전국적인 개입이 필요하다.
미국에서는 CDC가 클렙시엘라 종의 세균과 대장균(E. coli)에 대해서만 카바페넴 내성이나 카바페네메이스 생성 검출을 권장하고 있다. 이는 분자적 방법을 사용하지 않고 미생물학 실험실에서 검사를 수행하는 것을 용이하게 하며, 이 두 종류가 미국에서 발생하는 대부분의 CRE이기 때문이다. 이 항생제 내성 균주인 CRKP의 감염률을 가능한 한 낮게 유지하려면 효과적인 살균, 오염 제거 절차가 중요하다.
2016년 8월 중순, 네바다주 와슈군의 한 주민이 CRE(특히 폐렴막대균) 감염으로 인해 리노에 입원했다. 같은 해 9월 초, 그녀는 패혈성 쇼크에 걸려 사망했다. CDC 검사에서 밝혀진 바로는, 환자에서 분리된 균주는 최후의 수단인 콜리스틴을 포함하여 미국에서 사용 가능한 모든 26가지 항생제에 내성이 있었다.[31] 그녀는 오른쪽 넙다리뼈 골절과 이어진 넙다리뼈와 엉덩관절 감염으로 인해 2년 동안 인도에서 입원한 병력이 있었고, 이 기간 동안 이 내성 균주에 감염됐을 것으로 생각된다.[32][33][34]
환자 사이에 클렙시엘라 감염이 퍼지는 것을 방지하기 위해 의료인은 특정 감염 통제 조치를 따라야 한다.[18] 손 위생에 대한 엄격한 준수(바람직하게는 손이 더러워졌을 시 알코올 손 소독제 사용, 또는 손을 씻을 경우 비누와 물 사용)가 포함된다. 그람 음성 간균을 대상으로는 알코올 손 소독제가 효과적이다.[35] 클렙시엘라 관련 질환이 있는 환자가 있는 병실에 들어갈 때는 가운과 장갑을 착용한다. 또한 의료 시설은 클렙시엘라 확산을 방지하기 위해 엄격한 청소 절차를 따라야 한다.[18]
감염의 확산을 방지하기 위해 환자는 다음의 경우들을 포함하여 매우 자주 손을 씻어야 한다.
감염된 폐렴막대균이 약물 내성을 가지지 않은 경우 항생제로 치료할 수 있다. 폐렴막대균에 의한 감염은 항생제가 덜 효과적이기 때문에 치료가 어려울 수 있다. 그러한 경우, 미생물학 랩은 감염을 치료할 항생제를 결정하기 위해 검사를 시행해야 한다.[18] 클렙시엘라 폐렴의 보다 구체적인 치료법은 위의 문단에 나와 있다. 다제내성 클렙시엘라 종의 요로감염증에 대해 아미카신과 메로페넴의 병용 요법이 제안되었다.[36]
다제내성 폐렴막대균 균주는 실험실 검사 결과 파지를 복강, 정맥, 비강 투여했을 때 생체 내(in vivo)에서 사멸되었다.[37] 새로운 감염성 파지는 주위 환경에서 가져올 수 있기 때문에, 파지에 대한 내성은 항생제 내성만큼 문제가 되지 않을 것이다. 파지 요법은 항생제를 완전히 대체하기보다는, 항생제와 함께 사용하여 항생제 활성을 보완할 수 있다.[38]
폐렴막대균 또는 폐렴간균(학명: Klebsiella pneumoniae, 클렙시엘라 뉴모니아)은 그람 음성, 비운동성, 락토스 발효, 통성 혐기성, 막대 모양의 협막을 가지는 세균이다. 맥콘키 선택배지에서 점액성(mucoid)의 락토스 발효 세균으로 나타난다.
입, 피부, 장의 정상세균총에서 발견되지만, 흡인하면 인간과 동물의 폐, 특히 폐포에 파괴적인 변화를 일으켜 가래와 같은 피 묻은 갈색, 또는 노란색의 젤리 같은 점액을 만들 수 있다. 임상 환경에서 폐렴막대균은 장내세균과에 속한 클렙시엘라속의 가장 중요한 세균이다. 클렙시엘라속에 속한 다른 세균인 K. oxytoca 나 K. rhinoscleromatis는 인간 임상 표본에서도 존재가 입증되었다. 최근 몇 년 동안 클렙시엘라속에 속한 종들은 병원 감염에서 중요한 병원체로 떠올랐다.
토양에서 자연적으로 발생하며 약 30%의 균주가 혐기성 조건에서 질소 고정을 일으킬 수 있다. 이 때문에 자유 생활 질소 고정 세균으로서 질소 고정 시스템이 많이 연구되었다. 폐렴막대균이 농업에서 작물 수확량을 증가시키는 것으로 입증되었기 때문에, 농업적인 흥미의 대상이 된다.
클렙시엘라 옥시토카와 밀접한 관계에 있다. 폐렴막대균은 인돌 검사 음성이며 3-하이드록시부티레이트가 아닌 멜레치토스에서 자라는 능력으로 구별된다.