pt-BR
nomes no trilho de navegação

Panleukopénie, též zvaná kočičí mor, je silně virulentní onemocnění s inkubační dobou 2–10 dní postihující pouze kočky. Onemocnění mívá zpravidla velmi rychlý průběh a může připomínat otravu. Projevuje se současnými prudkými průjmy a zvracením, velmi často končí smrtí zvířete. Lze proti němu očkovat.
Nemoc způsobuje kočičí parvovirus, který napadá výstelku trávicího traktu, způsobuje vředy a rozpad výstelkového epitelu. Zároveň se snižuje množství bílých krvinek, což zhoršuje imunitu zvířete. Nákazu si mezi sebou zvířata většinou předávají přes sekrety, kterými se kontaminuje například voda k pití. Postižené zvíře je apatické, zvrací, má vodnatý průjem, mnohdy je ve stolici krev, nejí a nepije. Pokud je nákaza slabší, je možnost vyléčení. Nakazí-li se březí kočka, většinou potratí. Pokud se narodí živá koťata, mívají postižený mozeček, následkem čehož mají zhoršenou koordinaci pohybu, nebo mohou být i slepá.[1]
Panleukopénie, též zvaná kočičí mor, je silně virulentní onemocnění s inkubační dobou 2–10 dní postihující pouze kočky. Onemocnění mívá zpravidla velmi rychlý průběh a může připomínat otravu. Projevuje se současnými prudkými průjmy a zvracením, velmi často končí smrtí zvířete. Lze proti němu očkovat.
Nemoc způsobuje kočičí parvovirus, který napadá výstelku trávicího traktu, způsobuje vředy a rozpad výstelkového epitelu. Zároveň se snižuje množství bílých krvinek, což zhoršuje imunitu zvířete. Nákazu si mezi sebou zvířata většinou předávají přes sekrety, kterými se kontaminuje například voda k pití. Postižené zvíře je apatické, zvrací, má vodnatý průjem, mnohdy je ve stolici krev, nejí a nepije. Pokud je nákaza slabší, je možnost vyléčení. Nakazí-li se březí kočka, většinou potratí. Pokud se narodí živá koťata, mívají postižený mozeček, následkem čehož mají zhoršenou koordinaci pohybu, nebo mohou být i slepá.
Die Panleukopenie ist eine häufig tödlich verlaufende, virusbedingte Katzenkrankheit. Sie wird auch als Katzenseuche, Katzenstaupe, infektiöse Enteritis der Katzen, Agranulomatose, Aleukozytose und Katzenpest bezeichnet. Erreger ist ein Virus aus der Gattung Parvovirus (von lat. parvus – klein). Die Krankheit ist eng verwandt mit der Parvovirose des Hundes und der infektiösen Panleukopenie der Marderartigen. Bei Menschen kann das verwandte, aber wesentlich weniger gefährliche Parvovirus B19 Erkrankungen auslösen.
Auslöser der Erkrankung ist ein Virus der Gattung Parvovirus, das Feline Panleukopenie-Virus (FPV oder FPLV) mit einer Größe von etwa 18 bis 26 Nanometern Durchmesser. Die im Virus verschlüsselten Erbinformationen sind zu 99 % mit denen des caninen Parvovirus identisch. Die Vermehrung des Virus findet im Zellkern der betroffenen Zelle statt und benötigt hierbei Funktionen, die nur während der Zellteilung vorliegen. Der Erreger ist gegenüber Umwelteinflüssen sehr unempfindlich. Bei Raumtemperatur bleibt er über ein Jahr infektiös, die meisten handelsüblichen Desinfektionsmittel vermögen ihn nicht zu inaktivieren. Zu den gegen das Virus wirksamen Substanzen zählen Natriumhypochlorit, Formaldehyd und Glutaraldehyd.
Der Erreger kommt weltweit und in allen Katzenpopulationen endemisch vor. Die Krankheit kann alle Arten der Familie der Katzen (Felidae) und darüber hinaus einige Kleinbären (Waschbär, Südamerikanischer Nasenbär), Katzenfrette und Nerze befallen.
Von der Krankheit werden vor allem noch nicht immunkompetente Jungtiere betroffen. Bei Hauskatzen tritt sie am häufigsten im Alter von drei bis fünf Monaten auf.
Der Erreger dringt über Kontakt mit infektiösem Material (Kot, Nasensekret, Urin) durch die Nasen- und Maulschleimhaut in den Körper ein. Die Inkubationszeit beträgt 2 bis 10 Tage. Da das Virus zur Vermehrung Zellen mit hoher Teilungsrate benötigt, befällt es besonders die sich fortwährend stark regenerierenden Zellen des Darmepithels, des Knochenmarks und des Lymphsystems.
Feten können über die Plazenta bereits im Mutterleib infiziert werden.
Die Symptome können sehr variabel ausgeprägt sein. Einige Tiere können sogar ohne vorherige Krankheitszeichen sterben (perakuter Verlauf).
Entsprechend den befallenen Organsystemen dominieren vor allem Symptome des Magen-Darm-Traktes und des Abwehrsystems. Neben dem Auftreten starker, oftmals blutiger Durchfälle kommt es zu einer starken Abnahme weißer Blutkörperchen (Leukopenie) und damit einer Verminderung der Abwehrfähigkeit des erkrankten Organismus, der daher für bakterielle Sekundärinfektionen besonders empfänglich ist.
Neben diesen Symptomen zeigen die betroffenen Tiere häufig Mattigkeit, Fressunlust, Dehydratation, Fieber, Nasenausfluss, Bindehautentzündung und Erbrechen. Mit dem Kot werden große Mengen hochinfektiösen Erregermaterials ausgeschieden.
Pränatale und perinatale Infektionen führen zu einer Kleinhirn-Ataxie.
Eine Verdachtsdiagnose liefern fehlende Impfung, Alter, klinische Symptome, der charakteristische Verlauf und eine schwere Leukopenie. Eine sichere Diagnose kann nur labordiagnostisch erstellt werden.
Ein wesentliches diagnostisches und prognostisches Kriterium ist die Anzahl der Leukozyten, die bei typischen Verläufen auf Werte um 2000 bis 4000 pro Mikroliter absinken. Liegt der Wert unter 1500, besteht eine schlechte Prognose.
Das Virus kann elektronenmikroskopisch im Kot nachgewiesen werden. Es gibt darüber hinaus Schnelltests zum Virusnachweis im Kot, die zwar eine hohe Spezifität, aber nur eine Sensitivität zwischen 50 und 80 % haben.[1] Bei nicht geimpften Katzen kann darüber hinaus ein Antikörpernachweis im Blut hilfreich sein.
Histopathologische Untersuchungen von Dünndarm, Lunge, Niere, Lymphknoten und Milz sowie Kleinhirn von abortierten Feten können Klärung bringen. Intranukleäre (im Zellkern befindliche) Einschlusskörperchen vom Typ B in Darmepithelzellen sind typisch. Weitere Symptome am Darm sind Nekrosen der Darmkrypten, Verlust der Darmzotten und der Lamina propria. Eine Kleinhirn-Hypoplasie ist typisch für infizierte Feten. Der Fluoreszenznachweis von Antikörpern in Dünndarm- und Milzproben ist ebenfalls sicheres Indiz.
Differentialdiagnostisch müssen Fremdkörper im Darm, Feline infektiöse Peritonitis, Feline Coronavirusinfektion (FECV), Katzenleukämie, Feline Herpesvirusinfektion, Feline Calicivirusinfektion und das Immundefizienzsyndrom der Katzen berücksichtigt werden.
Die Behandlung erkrankter Tiere zielt zunächst auf eine Stabilisierung des Patienten hin. Hierzu sind meist Infusionen nötig um eine Austrocknung (Dehydratisierung) zu verhindern und eine optimale Ernährung zu gewährleisten. Um bakterielle Infektionen zu vermeiden, bedarf es der Verabreichung von Antibiotika. Das Virus selbst kann durch die Applikation von Interferonen und Serum-Antikörpern bekämpft werden. Bei intensiver Behandlung lassen sich die meisten Tiere retten.
Ein weiterer wesentlicher Aspekt der Krankheit ist die Einhaltung strikter Hygienemaßnahmen, um die Weiterverbreitung des Erregers zu verhindern. Genesende Katzen können das Virus bis zu sechs Wochen ausscheiden.
Die wirksamste Maßnahme gegen die Erkrankung besteht in einer prophylaktischen Impfung, welche erstmals im Alter von acht Wochen durchgeführt wird und nach einem Monat aufgefrischt werden sollte. In der Folge sind Impfintervalle von ein bis drei Jahren empfohlen. Nur mittels einer Impfung lässt sich die Infektion einer Katze sicher vermeiden.
Da bei regelmäßig geimpften Mutterkatzen die Katzenwelpen oft noch sehr viele mütterliche Antikörper haben, wird seit Juli 2006 eine dritte Auffrischungsimpfung im vierten Lebensmonat empfohlen. Mütterliche Antikörper können bis zu einem Lebensalter von 20 Wochen erhalten bleiben und offenbar reichen bereits geringe Mengen dieser Antikörper aus, um einen ausreichenden Impfschutz zu verhindern. Allerdings gibt es hinsichtlich der Schutzwirkung der Erstimpfungen auch Differenzen zwischen den Impfstoffen verschiedener Hersteller. Für eine bessere Einschätzung der Wirksamkeit der ersten Impfung ist eine vorherige Bestimmung des Antikörpertiters bei der Mutterkatze oder der maternalen Antikörper bei den Welpen empfehlenswert.[2]
Danach erfolgt eine Impfung nach einem Jahr, womit die Grundimmunisierung abgeschlossen ist, und erst dann kann auf ein Intervall von drei Jahren ausgedehnt werden. Allerdings wird für Zuchtstätten und Tierheime weiterhin empfohlen, die Auffrischungsimpfung jährlich durchführen zu lassen. Hier kann der Infektionsdruck höher sein, da in Zuchtstätten öfter Stresssituationen auftreten, mehrfach Neuzugänge hinzukommen und Besucher Parvoviren mitbringen können.[3]
Bei Lebendimpfstoffen beginnt der Schutz zwei Wochen nach der Grundimmunisierung. Lebendimpfstoffe dürfen aber nicht bei trächtigen Katzen oder Katzenwelpen unter vier Wochen eingesetzt werden. Hier muss auf Totimpfstoffe zurückgegriffen werden.
Die Panleukopenie ist eine häufig tödlich verlaufende, virusbedingte Katzenkrankheit. Sie wird auch als Katzenseuche, Katzenstaupe, infektiöse Enteritis der Katzen, Agranulomatose, Aleukozytose und Katzenpest bezeichnet. Erreger ist ein Virus aus der Gattung Parvovirus (von lat. parvus – klein). Die Krankheit ist eng verwandt mit der Parvovirose des Hundes und der infektiösen Panleukopenie der Marderartigen. Bei Menschen kann das verwandte, aber wesentlich weniger gefährliche Parvovirus B19 Erkrankungen auslösen.
Carnivore protoparvovirus 1 is a species of parvovirus that infects carnivorans. It causes a highly contagious disease in both dogs and cats separately. The disease is generally divided into two major genogroups: FPV containing the classical feline panleukopenia virus (FPLV), and CPV-2 containing the canine parvovirus type 2 (CPV-2) which appeared in the 1970s.[2]
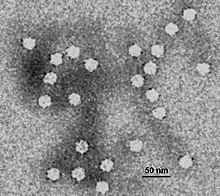
Belonging to the family Parvoviridae, FPLV have linear, single-stranded DNA (ssDNA) genomes. This agent is one of the smallest animal viruses, barely 18 to 20 nm in diameter.[3] Like other parvovirus genomes, it has hairpin structures at both ends of its genome: 3-genome Y-type structure and 5-terminal U-shaped structure, making it challenging to amplify the full-length genome of parvovirus despite its small size.[4] Sequences in the genome show a high degree of nucleotide conservation in the VP2 gene after over 90 years since it has emerged; the VP2 gene codes for the capsid protein VP2, a main structural protein, which determines the major mutations during the evolution of CPV.[5]
FPLV is known to infect all wild and domestic members of the felid (cat) family worldwide.[6] It is a highly contagious, severe infection that causes gastrointestinal, immune system, and nervous system disease. Its primary effect is to decrease the number of white blood cells, causing the disease known as feline panleukopenia.
Although it was once thought that only FPLV infects cats,[7] it has been confirmed that a feline panleukopenia illness can be caused by CPV 2a, 2b, and 2c.[8][9]
FPLV is commonly referred to as:
It is sometimes confusingly referred to as "cat plague" and "feline distemper".[11]
In addition to members of the felid family, it can also affect other carnivorans (e.g. raccoon, mink).[6]
Formed within English using elements derived from Greek: pan- a combining form meaning "universal" or "worldwide", -leuco- a combining form meaning (in biologic sciences) "white blood cell", and -penia a combining form meaning "loss of" or "decrease of". Thus the word means universal loss of white blood cells. The universal part refers to both its worldwide distribution and to the fact that all species of cats are infected.[12]
The feline panleukopenia virus is considered ubiquitous, meaning it is in virtually every place that is not regularly disinfected. The infection is highly contagious among unvaccinated cats.[13]
Antibodies against FPLV, produced by the adaptive immune system, play an important role in the feline response to the virus. Maternally-derived antibodies (MDA) efficiently protect kittens from fatal infection. This passively acquired immunity is later replaced by an active immune response obtained by vaccination or as a consequence of a natural infection.[14] In kittens, the period of greatest susceptibility to infection is when maternal antibodies are absent, or waning, and vaccine-induced immunity has not yet fully developed.[15]
Free-roaming cats are thought to be exposed to the virus during their first year of life. Those that develop a subclinical infection or survive acute illness mount a robust, long-lasting, protective immune response.[6]
An infected cat sheds large amounts of virus in all body secretions including feces, vomit, urine, saliva, and mucus during the acute phase of illness. It can continue to shed the virus for as long as six weeks after recovery.[6] Subclinically ill cats can also shed the virus in body secretions. The virus can be carried or transferred on an infected object (such as bedding, food dishes, fur) or by other animals, fleas, and humans [16] (see: fomites). It persists long after evidence of the original body secretion has faded away, and can be transported long distances. Like all parvoviruses, FPLV is extremely resistant to inactivation and can survive for longer than one year in a suitable environment.[17] Kitten deaths have been reported in households of fully vaccinated cats, possibly because of exposure to large amounts of virus in the environment.[18][19] In a recent study, microRNA responses to FPLV infection were identified in feline kidney cells by sequencing, providing a possible link between miRNA expression and pathogenesis of FPV infection.[20]
Infection occurs when the virus enters the body through the mouth or nose. Whether illness results or not depends on the immunity in the victim vs. the number of individual virus particles (i.e. the amount of virus) entering the body.[13]
The clinical manifestations of FPLV are variable based on the dose of the virus, the age of the cat, potential breed predispositions, and prior immunity from maternal antibodies, previous exposure, or vaccination.[21] Most infections are subclinical, as evidenced by the high seroprevalence of anti-FPV antibodies among some populations of unvaccinated, healthy cats. The cats that become clinically ill are usually less than one year old, but older cats are also at risk.[22][23] There is high mortality in clinically affected kittens and sudden death can occur.[21]
Clinical signs usually develop in 4–6 days after exposure, but can show in 2–14 days.[24] The virus infects and destroys actively dividing cells in bone marrow, lymphoid tissues, intestinal epithelium, and—in very young animals—in the cerebellum and retina.[6] The virus primarily attacks the lining of the gastrointestinal tract, causing internal ulceration and, ultimately, total sloughing of the intestinal epithelium.
Primary signs include:
Clinical laboratory findings include (but are not limited to):[15][22]
Other signs include: fever, loss of skin elasticity due to dehydration, abdominal pain, sternal recumbency with splayed legs and head droop, nasal discharge and conjunctivitis.[21] Cats may sit at a water bowl, but not drink.[6] Terminal cases are hypothermic and may develop septic shock and disseminated intravascular coagulation.[17]
Infection in pregnant cats can result in fetal resorption, mummification, abortion, or stillbirth of neonates.[6] Fetuses infected in utero that survive and kittens less than a few weeks of age that become infected can have cerebellar hypoplasia, retinal dysplasia, and optic neuropathy.[25]
A presumptive clinical diagnosis of FPLV can be made for kittens with appropriate signalment, history, clinical findings and the history of no prior vaccination.[21]
The clinical diagnosis is usually supported by documenting parvovirus antigen in feces by ELISA (enzyme-linked immunosorbent assay) and PCR (polymerase chain reaction) assays. The availability of validated assays varies by country but is becoming more common. PCR assays are so sensitive that FPV DNA can be amplified from feces of cats vaccinated with modified live strains of the virus. Attenuated parvoviruses in MLV vaccines replicate in the blood and intestine, and post-vaccinal fecal shedding of FPV has been demonstrated, which can result in recent vaccinations giving false positive results on diagnostic tests.[26] At least one of the ELISA antigen tests for dogs (SNAP®Parvo; IDEXX Laboratories) detects FPV in feline feces and has a cut point for a positive test result that excludes most vaccinated cats. Thus, this ELISA is superior to PCR for screening cats for FPV infection and can also be performed in the veterinary clinic. (These are only approved and licensed for detecting canine parvovirus, but it is generally known that they also detect FPL viral antigen in feline feces. These tests are used extra-label because they allow rapid, inexpensive, in-house detection of the virus.[27]) Positive fecal SNAP test results, including weak positives, are highly likely to be true positives in clinically affected animals.[28] Some cats will have completed the shedding period by the time the test is run, leading to false-negative results.[21][27][29] Electron microscopy, virus isolation and seroconversion can also be used to document active or recent infection.
Leukopenia on a complete blood count (nadir 50–3,000 WBC/μL) supports a diagnosis of FPLV. In an unvaccinated cat, the presence of antibodies against FPV indicates that the cat either has the disease or has had the disease in the past. Elevated IgM titers (1:10 or greater) indicate active infection and if clinical signs are obvious (diarrhea, panleukopenia) the prognosis is poor. Elevated IgG titers (1:100 or greater) in a cat with clinical signs indicates a better prognosis.[30]
Differential diagnoses include salmonellosis, enteric toxins, feline immunodeficiency virus (FIV), feline leukemia virus (FeLV), cryptosporidiosis, pancreatitis, septicaemia with acute endotoxemia, toxoplasmosis, peritonitis, and lymphoma.[30]
To contain the virus, cats with suspected or diagnosed FPLV should be kept in isolation.[31]
It requires immediate, aggressive treatment if the cat is to survive, as it can be fatal in less than 24 hours. Several articles and publications provide guidance for rescuers and veterinarians for optimizing outcomes.[32][31]
Treatment involves:[33]
Feeding should be continued as long as possible.[34] A highly digestible diet is preferred, but the individual animal's preferences may dictate giving whatever it will eat.[31] In anorexic, hypoproteinemic, vomiting and diarrheic cats parenteral nutrition is required.[32]
In a disease outbreak, unvaccinated kittens or adults can be given anti-FPV serum containing FPV antibodies injected subcutaneously or intraperitoneal. This may provide protection for 2–4 weeks.[35][36] Therapeutic efficacy of anti-FPV serum has been demonstrated in dogs,[37] and similar beneficial effects may be expected in cats.[31]
Several studies have shown recombinant feline interferon omega is effective in the treatment of parvoviral enteritis in dogs[38][39] and also inhibits replication of FPV in cell culture. So far no data are available on its efficacy in FPV-infected cats.
Cats typically die due to complications associated with sepsis, dehydration, and disseminated intravascular coagulopathy (DIC).[32] Leukocytopenia predisposes patients to secondary infections, especially bacterial and fungal, though secondary viral infections also occur.
It has been stated that cats with FPLV may be at risk for endocarditis or cardiomyopathy (since CPV-2 is a well-known cause of viral myocarditis in young puppies), but a 2017 retrospective study concluded that "Feline Panleukopenia Virus Is Not Associated With Myocarditis or Endomyocardial Restrictive Cardiomyopathy in Cats".[40]
Mortality in affected felid litters varies between 20 and 100%.[41] Mortality of FPLV is 25–90% in domestic cats with the acute form of the disease and up to 100% in cats with peracute disease.[32][42]
In 2010, a retrospective study of 244 infected cats showed that "leukocyte and thrombocyte counts as well as serum albumin and potassium concentrations at presentation are prognostic indicators in cats with panleukopenia, whereas vaccination status, age, clinical signs, and housing conditions are not."[23]
A survival rate of about 50% has been reported with supportive therapies.[43] Cats with FPLV that survive the first five days of treatment usually recover;[29] however, the decrease in the cat's white blood cells compromises its immune system, leaving it vulnerable to secondary infection.[44]
Lifelong immunity is thought to follow recovery from disease, and a carrier state of the disease has never been identified.[15]
Cats with suspected or diagnosed FPLV should be kept in isolation. This non-enveloped virus is very resistant to environmental conditions and many disinfectants, is highly contagious, and rapidly accumulates in the environment due to high shedding of virus from affected animals.[45] Strict protocols for containment – with isolation, minimal handling, and disinfection of all potential sources of fomites – is warranted. Recovered cats can still shed the virus for up to six weeks[6] and can carry it on their body for prolonged periods.
The practice of recommending and giving vaccines on a fixed schedule with annual boosters has been widely discarded. Current recommendations are based on the philosophy of vaccinating each cat no more frequently than necessary. These recommendations take into account considerations for the efficacy and longevity of each specific vaccine; the exposure, risk, and need of different cat populations; and socioeconomic limitations.[46][47][48][49]
Recommendations vary for:
The FPLV vaccination is considered a "core" (essential for health) vaccine and is recommended for all domestic cats.[46][50] Even cats kept indoors can be infected from fomite transmission.[51]
Several types and brands of commercial FPLV vaccines are available to induce acquired immunity. These include:
Combination vaccines that protect against several common viruses, including FPLV, are also available.
Selection or use of a specific type/brand of a vaccine may vary depending on the overall risk of viral infection to the specific animal in its environment, along with considerations for the time it takes to confer protection, its overall efficacy, the animal's health, and the potential risks associated with MLV vs killed, adjuvanted vs nonadjuvanted, intranasal/ocular vs injection.
Modified-live FPLV vaccines are not recommended in pregnant queens, very young kittens, or cats with FIV or FeLV.[52][46]
Kittens without maternally derived antibodies are especially vulnerable. FPLV vaccination can start as early as 4 weeks of age for kittens at high risk but are usually started at 6 weeks, then given every 3–4 weeks until 16 weeks of age. For cats older than 16 weeks, 2 doses, 3 to 4 weeks apart is generally recommended, followed by a 6-month to 1-year booster.[47][46] Thereafter, a booster vaccination every 3 years is usually recommended;[53][46] a blood titer test can be done to determine individual antibody levels for catering the timing of boosters.
Carnivore protoparvovirus 1 is a species of parvovirus that infects carnivorans. It causes a highly contagious disease in both dogs and cats separately. The disease is generally divided into two major genogroups: FPV containing the classical feline panleukopenia virus (FPLV), and CPV-2 containing the canine parvovirus type 2 (CPV-2) which appeared in the 1970s.
Belonging to the family Parvoviridae, FPLV have linear, single-stranded DNA (ssDNA) genomes. This agent is one of the smallest animal viruses, barely 18 to 20 nm in diameter. Like other parvovirus genomes, it has hairpin structures at both ends of its genome: 3-genome Y-type structure and 5-terminal U-shaped structure, making it challenging to amplify the full-length genome of parvovirus despite its small size. Sequences in the genome show a high degree of nucleotide conservation in the VP2 gene after over 90 years since it has emerged; the VP2 gene codes for the capsid protein VP2, a main structural protein, which determines the major mutations during the evolution of CPV.
FPLV is known to infect all wild and domestic members of the felid (cat) family worldwide. It is a highly contagious, severe infection that causes gastrointestinal, immune system, and nervous system disease. Its primary effect is to decrease the number of white blood cells, causing the disease known as feline panleukopenia.
Although it was once thought that only FPLV infects cats, it has been confirmed that a feline panleukopenia illness can be caused by CPV 2a, 2b, and 2c.
FPLV is commonly referred to as:
feline infectious enteritis virus (FIE) feline parvovirus (FPV or FP or "feline parvo") feline parvoviral enteritisIt is sometimes confusingly referred to as "cat plague" and "feline distemper".
In addition to members of the felid family, it can also affect other carnivorans (e.g. raccoon, mink).
Kissarutto on kissoissa esiintyvä feline panleukopenia -parvoviruksen aiheuttama tartuntatauti. Se on erittäin tarttuva, ja sen itämisaika on 2–10 vuorokautta. Kissa, jolla on todettu tai epäillään kissaruttoa, tulisi eristää muista kissoista. Tauti tarttuu eritteiden kautta, ja syntymättömät pennut voivat saada sen emoltaan. Sikiössä parvovirus voi aiheuttaa pikkuaivojen vajaakehitystä. Sairastuneesta kissasta tulee ruokahaluton ja apaattinen, ja se alkaa oksennella ja ripuloida, mikä johtaa kissan kuivumiseen. Kuolleisuus tautiin on suuri, ja ainoa keino tautia vastaan on rokottaminen. Mikäli kissa selviytyy taudista hengissä, se saa siihen elinikäisen immuniteetin.
Kissaruton aiheuttaja on parvoviruksiin kuuluva kissaruttovirus (FPLV, Feline Panleucopenia Virus). Se on hyvin ympäristöolosuhteita ja kemikaaleja kestävä vaipaton DNA-virus, joka säilyy kuukausia tartuntakykyisenä viruksella saastuneessa ympäristössä. Sairastuneet eläimet erittävät sitä runsaasti ulosteissaan. Virus tarttuu helposti, ja tartunnalle alttiit eläimet saattavat saada tartunnan saastuneesta ympäristöstä jopa puhdistuksen ja desinfektion jälkeen.
Kissaruttovirus leviää sairaan kissan ulosteiden välityksellä ja tarttuu suun kautta. Virus lisääntyy ensin nenänielussa ja leviää sitten verenkierron välityksellä lähes kaikkialle kissan elimistöön. Viruksen lisääntyminen vaatii aktiivisesti jakautuvaa solukkoa. Virus iskee mm. immuunijärjestelmän soluihin, mistä on seurauksena immuunivajavuus. Lymfosyyttien määrän lasku voi johtua suoraan niiden tuhoutumisesta ja epäsuorasti niiden vaeltamisesta kudoksiin. Luuydin on myös viruksen kohteena, mikä selittää taudille tyypillisen valkosolujen määrän dramaattisen laskun.
Kissaruton tyypillinen oire on ripuli, joka johtuu suolistonukan vaurioitumisesta viruksen tuhotessa suolen pintasolukkoa. Virus lisääntyy nopeasti jakautuvissa suolen sisäpinnan soluissa. Samanaikainen muu virusinfektio, kuten kissan koronavirus, saattaa pahentaa tautia entisestään. Kissarutto voi tarttua kaikenikäisiin kissoihin. Pennut ovat tartunnalle kaikkein herkimpiä, ja niillä tauti on myös vakavin – yli 90 % tartunnan saaneista menehtyy. Kohdussa tai pian syntymän jälkeen saatu infektio voi vaikuttaa keskushermostoon johtaen pikkuaivojen vaurioitumiseen, joka ilmenee liikehäiriöinä, esim. haparoivana liikkumisena, kaatuiluna ja tärinänä.
Kissarutto voidaan todeta osoittamalla virus ulosteesta kaupallisilla pikatesteillä. Näiden testien herkkyys ja tarkkuus on riittävä verrattuna PCR-testaukseen tai perinteiseen viruksen eristämiseen ja viljelyyn soluviljelmissä. Seerumin vasta-aineita voidaan osoittaa erilaisilla serologisilla testeillä.
Käyttökelpoista spesifistä hoitoa ei ole tarjolla, joten sairaan kissan hoito perustuu sen elintoimintojen tukemiseen ja sekundaaristen bakteeritulehdusten torjuntaan. Suonensisäinen nesteytys, jolla pyritään korjaamaan ripulin aiheuttamat neste- ja suolatasapainon häiriöt, on tärkein oireenmukainen hoito. Suolenseinämän vakava vaurioituminen virusinfektion seurauksena voi johtaa siihen, että suolensisällön bakteerit pääsevät tunkeutumaan verenkiertoon. Tämä voi helposti aiheuttaa verenmyrkytyksen, koska sairaan kissan immuunijärjestelmä on myös viruksen runtelema. Verenmyrkytyksen ennaltaehkäisyyn käytetään antibioottihoitoa, joka annetaan usein suonensisäisesti.
Koska kissarutto on usein vakava ja virus on niin yleinen ja kestävä ympäristössä, kaikkien kissojen rokottamista sitä vastaan suositellaan vahvasti. Sisäkissatkaan eivät ole suojassa tartunnalta, koska virus voi siirtyä sisätiloihin esim. omistajan kengissä.
Kissarutto on kissoissa esiintyvä feline panleukopenia -parvoviruksen aiheuttama tartuntatauti. Se on erittäin tarttuva, ja sen itämisaika on 2–10 vuorokautta. Kissa, jolla on todettu tai epäillään kissaruttoa, tulisi eristää muista kissoista. Tauti tarttuu eritteiden kautta, ja syntymättömät pennut voivat saada sen emoltaan. Sikiössä parvovirus voi aiheuttaa pikkuaivojen vajaakehitystä. Sairastuneesta kissasta tulee ruokahaluton ja apaattinen, ja se alkaa oksennella ja ripuloida, mikä johtaa kissan kuivumiseen. Kuolleisuus tautiin on suuri, ja ainoa keino tautia vastaan on rokottaminen. Mikäli kissa selviytyy taudista hengissä, se saa siihen elinikäisen immuniteetin.
Le typhus félin, aussi appelé panleucopénie féline, est une maladie infectieuse du chat particulièrement contagieuse et potentiellement mortelle provoquée par un parvovirus, qui affecte également les ratons-laveurs et les visons.
Le mot typhus vient du grec tuphos, qui signifie stupeur extrême, prostration.
Les chatons sont les plus susceptibles de contracter le typhus félin. La mortalité atteint ainsi 90 % chez les jeunes chats. Les lieux avec de fortes populations de chats (élevages, refuges) sont également plus susceptibles de voir la maladie se déclarer[1].
La maladie peut affecter le fœtus d'une chatte gestante et avoir comme conséquences une ataxie cérébrale ou bien la mort du petit[1].
Le virus se transmet par voie oro-nasale et fécale de manière indirecte. Le virus est également très résistant à plusieurs désinfectants. Un environnement contaminé par un individu peut ainsi le rester pendant plusieurs mois[1].
Le typhus félin est provoqué par un virus de type parvovirus non enveloppé à ADN simple brin[2], qui est particulièrement résistant dans l'environnement[1].
Le typhus provoque une infection systémique. Le virus utilise la machinerie cellulaire afin de se répliquer dans l'organisme. En s'attaquant aux tissus lymphoïdes, le virus détruit les lymphocytes et ainsi engendrer une immunosuppression[1]. Le virus peut provoquer une entérite en attaquant les cellules intestinales des cryptes de Lieberkühn, générant des diarrhées. En pénétrant la moelle osseuse, une neutropénie peut survenir[1].
Les antigènes viraux du typhus félin peuvent être détectés à partir des fèces par un test d'agglutination ou bien par immunochromatographie. Un test PCR réalisé en laboratoire à partir d'un échantillon de fèces ou de sang entier peut également être employé[1].
Cette maladie se caractérise par une gastro-entérite accompagnée d'un état de tuphos (profond abattement), et d'une panleucopénie (diminution de tous les leucocytes), d'où son synonyme de panleucopénie féline.
Les symptômes suivants peuvent être observés :
L'animal doit tout d'abord être isolé en raison de sa contagiosité importante. Le traitement consiste surtout en un traitement de soutien : maintien de l'hydratation (perfusion), de l'équilibre acido-basique de l'organisme, antibiotique à large spectre pour éviter toute septicémie[1]. En cas de vomissements, un antiémétique est administré afin de permettre à l'animal de s'alimenter. Il est possible de lui donner un aliment hautement digestible associé à des vitamines.
La vaccination contre le typhus félin est recommandé pour tous les chats, y compris les ceux qui n'ont pas accès à l'extérieur car ils peuvent également être contaminés par le virus[1].
Le chaton est normalement protégé par les anticorps maternels pendant 6 à 8 semaines après sa naissance. Par la suite, le centre européen des maladies félines (Advisory Board on Cat Diseases) recommande de les vacciner entre 8 et 9 semaines, puis d'effectuer un rappel 3 à 4 semaines plus tard, à un minimum 12 semaines d'âge[1].
Le typhus félin, aussi appelé panleucopénie féline, est une maladie infectieuse du chat particulièrement contagieuse et potentiellement mortelle provoquée par un parvovirus, qui affecte également les ratons-laveurs et les visons.
Le mot typhus vient du grec tuphos, qui signifie stupeur extrême, prostration.
Panleukopenia kucing (bahasa Inggris: Feline panleukopenia virus, disingkat FPV) adalah infeksi virus yang menyerang kucing, baik kucing liar maupun peliharaan. Penyakit ini disebabkan oleh parvovirus kucing yang merupakan kerabat dekat parvovirus anjing tipe 2 dan enteritis cerpelai. Penyakit ini sangat menular dan dapat membunuh kucing yang terinfeksi.[1] Nama "panleukopenia" mengacu pada rendahnya jumlah sel darah putih (leukosit) pada kucing yang terserang penyakit ini.[2]
Seekor kucing dapat tertular panleukopenia jika berhubungan dengan cairan tubuh atau tinja kucing yang tertular, objek-objek lain yang dapat membawa virus panleukopenia, dan kutu.[2] Panleukopenia bahkan dapat disebarkan oleh selimut, piring, atau pakaian dan sepatu orang yang pernah bersentuhan dengan kucing yang terinfeksi. Seperti parvovirus-parvovirus lainnya, virus panleukopenia kucing dapat bertahan selama lebih dari satu tahun di lingkungan yang tepat.[1] Namun, penyakit ini tidak dapat menulari manusia.[2]
Virus panleukopenia kucing menyerang saluran pencernaan kucing dan memicu ulkus peptikum. Akibatnya, terjadi diare yang berdarah, dehidrasi, malnutrisi, anemia, dan bahkan kematian. Jumlah sel darah putih juga berkurang, sehingga sistem kekebalan tubuh melemah. Selain itu, jumlah hematokrit dan platelet turut berkurang. Gejala-gejala lain meliputi depresi, rasa lesu, hilangnya nafsu makan, demam, muntah, kulit tidak lagi elastis akibat dehidrasi, dan perilaku menggigit ekor, punggung belakang, dan kaki belakang sendiri. Sebagian besar kucing yang terinfeksi mati karena dehidrasi yang disebabkan oleh diare atau infeksi sekunder yang dipicu oleh kelemahan sistem kekebalan tubuh.[3]
Setiap kucing yang sehat sebaiknya diberi vaksin panleukopenia, termasuk kucing rumahan karena virus ini dapat bertahan lama dan disebarkan lewat benda-benda.[4] Bila seekor kucing terserang penyakit ini saat sedang hamil, virus panleukopenia kucing dapat menyebabkan hipoplasia otak kecil pada anak-anaknya. Maka dari itu, vaksin panleukopenia kucing tidak boleh diberikan kepada kucing yang sedang hamil.
Penyakit ini perlu ditangani secara agresif karena kucing yang tertular dapat kehilangan nyawanya dalam waktu kurang dari 24 jam. Penanganan yang dapat dilakukan adalah transfusi darah untuk meningkatkan pansitopenia, infus cairan untuk menangani dehidrasi, dan pemberian vitamin A, B, dan C, serta antibiotik IV untuk menangani septisemia. Kucing yang tertular penyakit ini juga harus diisolasi di rumah sakit.[5]
Panleukopenia kucing (bahasa Inggris: Feline panleukopenia virus, disingkat FPV) adalah infeksi virus yang menyerang kucing, baik kucing liar maupun peliharaan. Penyakit ini disebabkan oleh parvovirus kucing yang merupakan kerabat dekat parvovirus anjing tipe 2 dan enteritis cerpelai. Penyakit ini sangat menular dan dapat membunuh kucing yang terinfeksi. Nama "panleukopenia" mengacu pada rendahnya jumlah sel darah putih (leukosit) pada kucing yang terserang penyakit ini.
Seekor kucing dapat tertular panleukopenia jika berhubungan dengan cairan tubuh atau tinja kucing yang tertular, objek-objek lain yang dapat membawa virus panleukopenia, dan kutu. Panleukopenia bahkan dapat disebarkan oleh selimut, piring, atau pakaian dan sepatu orang yang pernah bersentuhan dengan kucing yang terinfeksi. Seperti parvovirus-parvovirus lainnya, virus panleukopenia kucing dapat bertahan selama lebih dari satu tahun di lingkungan yang tepat. Namun, penyakit ini tidak dapat menulari manusia.
Virus panleukopenia kucing menyerang saluran pencernaan kucing dan memicu ulkus peptikum. Akibatnya, terjadi diare yang berdarah, dehidrasi, malnutrisi, anemia, dan bahkan kematian. Jumlah sel darah putih juga berkurang, sehingga sistem kekebalan tubuh melemah. Selain itu, jumlah hematokrit dan platelet turut berkurang. Gejala-gejala lain meliputi depresi, rasa lesu, hilangnya nafsu makan, demam, muntah, kulit tidak lagi elastis akibat dehidrasi, dan perilaku menggigit ekor, punggung belakang, dan kaki belakang sendiri. Sebagian besar kucing yang terinfeksi mati karena dehidrasi yang disebabkan oleh diare atau infeksi sekunder yang dipicu oleh kelemahan sistem kekebalan tubuh.
Setiap kucing yang sehat sebaiknya diberi vaksin panleukopenia, termasuk kucing rumahan karena virus ini dapat bertahan lama dan disebarkan lewat benda-benda. Bila seekor kucing terserang penyakit ini saat sedang hamil, virus panleukopenia kucing dapat menyebabkan hipoplasia otak kecil pada anak-anaknya. Maka dari itu, vaksin panleukopenia kucing tidak boleh diberikan kepada kucing yang sedang hamil.
La panleucopenia felina (FPV), anche denominata gastroenterite felina o tifo felino è una malattia infettiva virale che colpisce il gatto e specie feline selvatiche[1][2].
È causata dal parvovirus felino (FPV), patogeno appartenente alla famiglia dei Parvoviridae a DNA monocatenario, privo di envelope, virus citolitico strettamente correlato al parvovirus canino di tipo 2 (CPV2). Questi possiedono un'identità aminoacidica pari al 95%, si differenziano per 8 sostituzioni aminoacidiche a livello della VP2 (proteina capsidica coinvolta in fenomeni immunitari, di virulenza e assorbimento).FPV è un precursore di CPV2; le quattro sostituzioni aminoacidiche avvenute a livello della porzione aminoterminale della VP2, coinvolta nel legame con la cellula ospite a livello di transferrina, hanno portato ad una variazione del tropismo con salto di specie (rendendo il virus in grado di infettare il cane); le altre quattro mutazioni sono avvenute a livello degli epitopi immunodominanti, con la creazione di un altro virus in grado di infettare il cane ma non il gatto (i due virus in minima parte cross-reagiscono). Queste mutazioni sono avvenute in carnivori selvatici (volpe, lupo) in maniera costante, portando alla formazione di sequenze FPV-like e CPV2-like. L'incidenza del CPV2 si è ridotta, in quanto sostituito da 3 varianti: 2a, 2b e 2c dalle quali si differenzia per 6 sostituzioni aminoacidiche. Queste invece, si differenziano tra loro per una sostituzione aminoacidica in posizione 426: 2a, asparagina 2b, acido aspartico 2c, acido glutammico Distinguibili mediante Ac monoclonali, sono in grado di infettare il gatto (a differenza del CPV-2 originale). FPV, è privo di enzimi di replicazione, (così come CPV2), motivo per cui replica nel nucleo delle cellule infette, sfruttandone gli enzimi di replicazione.
Il virus della panleucopenia felina è principalmente trasmesso attraverso il contatto con fluidi corporei di altri felini, ma anche grazie a vettori quali le pulci.[2] Non è trasmissibile all'uomo. Come tutti i parvovirus, è estremamente resistente nell'ambiente e può sopravvivere anche un anno in un ambiente adatto.
L'enterite da parvovirus del gatto - analogamente al cane - è una malattia grave, spesso letale. Il principale bersaglio del virus sono le cellule in attiva replicazione: le cripte epiteliali del tratto gastroenterico ed il midollo osseo.[3]
Le lesioni precoci della malattia comprendono una deplezione linfoide e l'involuzione del timo. Le lesioni gastroenteriche sono generalmente limitate all'intestino tenue, con ulcerazione, necrosi ed esfoliazione. Ne consegue una diarrea profusa e solitamente emorragica, con grave disidratazione, malnutrizione, anemia, e spesso morte. L'infiltrato infiammatorio cronico è presente nella lamina propria della mucosa intestinale.[3] Gran parte dei decessi da panleucopenia felina sono causati da una concomitanza di infezioni batteriche secondarie con endotossiemia.
L'infezione intrauterina provoca ipoplasia cerebellare nei gattini.[3]
I sintomi primari sono: vomito, febbre, anoressia, disidratazione, congestione e secchezza mucosa orale e faringea, dolore addominale, ingrossamento linfonodale, per poi seguitare con la leucopenia e la diarrea, portando rapidamente alla morte nella maggioranza dei casi[1]. Se la patologia colpisce una femmina gravida si possono, in alcuni casi, avere dei danni cerebrali al feto o anche l'aborto.
Esiste un vaccino per la prevenzione dell'infezione, che ha tuttavia dimostrato un'efficacia limitata nei cuccioli e non deve essere dato a una gatta gravida perché può dare problemi al feto.
Il virus della panleucopenia felina, a causa della sua specificità, morbosità e letalità è stato ed è talvolta impiegato per le operazioni di eradicazione felina dalle isole. In questi ambienti ecologici unici, sovente ricchi di endemismi e specie introvabili altrove, i gatti sono oramai da tempo riconosciuti come una delle principali cause d'estinzione delle specie autoctone, nonché come principale minaccia alla biodiversità e all'equilibrio biosistemico. Un esempio riuscito di tale approccio eradicativo è quello delle isole del principe Edoardo. Il virus, in soli cinque anni, ha ridotto a circa un sesto la popolazione felina alloctona. I restanti animali sono stati abbattuti dagli ecologisti, nel corso dei successivi dieci anni, mediante battute di caccia notturne fino al 1991 quando, almeno parzialmente, l'equilibrio naturale dell'isola fu finalmente ripristinato e la popolazione di gatti fu dichiarata ufficialmente azzerata.[4]
La panleucopenia felina (FPV), anche denominata gastroenterite felina o tifo felino è una malattia infettiva virale che colpisce il gatto e specie feline selvatiche.
Panleukopenia kotów (łac. Panleucopenia infectiosa felinum). Znana również jako: zakaźne zapalenie jelit kotów, tyfus koci, nosówka kotów (ang. Feline distemper), agranulocytoza.
Jest to zakaźna, wysoce zaraźliwa, wirusowa choroba wszystkich kotowatych. Przebiega z objawami zapalenia układu pokarmowego, wraz z towarzyszącą leukopenią. Choroba występuje na całym świecie.
Przyczyną jest wirus panleukopenii kotów (ang. feline panleukopenia virus – FPV). Jest on blisko spokrewniony z parwowirusem psów typ 2 (CPV-2). Wirus jest oporny na warunki środowiska zewnętrznego i większość środków dezynfekcyjnych. Najbardziej wrażliwe na zachorowanie są młode zwierzęta między 2 a 5 miesiącem życia, u których spadł poziom przeciwciał matczynych.
Źródłami zakażenia są wydzieliny i wydaliny chorych zwierząt (głównie kał, ślina, wymiociny). Zakażenie następuje najczęściej drogą pokarmową wirusem obecnym w środowisku (miski, kuwety, legowiska) oraz przez kontakt bezpośredni. Możliwe jest przeniesienie zakażenia przez owady (pchły, wszy itp.).
W pierwszym rzędzie wirus namnaża się w tkance limfoidalnej okolicy gardła. Po kilku dniach od zakażenia następuje wiremia, tą drogą zarazek przedostaje się do węzłów chłonnych, śledziony, grasicy, szpiku kostnego i krypt jelitowych. U ciężarnych kotek wirus przenika do komórek płodu i łożyska. Zaatakowanie komórek układu limfatycznego i szpiku skutkuje znaczną immunosupresją, co w konsekwencji daje powikłania w postaci wtórnych infekcji.
Okres wylęgania choroby wynosi zwykle od 4 – 10 dni. Dominujące objawy to: gorączka, depresja, biegunka (czasem z domieszką krwi), wymioty, odwodnienie, znaczna bolesność brzucha, wychudzenie. W obrazie krwi obserwujemy spadek ilości krwinek białych – leukopenia (panleukopenia, agranulocytoza). U młodych zwierząt może wystąpić nadostry przebieg choroby – śmierć następuje nagle, bez pojawienia się objawów. W przebiegu ostrym padnięcia następują najczęściej w 3 – 5 dniu choroby.
Współczynnik umieralności jest wysoki i bez podtrzymującej terapii może wynosić nawet do 75%[potrzebny przypis]. Śmiertelność nieszczepionych kociąt wynosi nawet do 90%[1]. Zwierzęta starsze, powyżej kilku miesięcy życia, chorują łagodniej, a śmiertelność jest dużo niższa.
Wirus FPV może przekroczyć łożysko, rezultatem czego może być resorpcja płodów, ronienia, wczesna śmierć noworodków, niedorozwój móżdżku potomstwa. Rzadko stan kociąt zaczyna się pogarszać dopiero w 2 – 3 tygodniu po porodzie, kiedy to zaczyna się u nich rozwijać brak koordynacji i ataksja.
Głównie obserwujemy: obraz nieżytowego zapalenia jelit cienkich wraz z nalotem włóknika (czasem zapalenie dyfteroidalno-martwicowe). Obrzęk nerek i wątroby. Krew w naczyniach nieskrzepła, ciemna. Żołądek pusty.
Objawy kliniczne uzupełnione badaniem obrazu krwi pozwalają z reguły na postawienie podejrzenia panleukopenii. Wirus można wykryć m.in. metodą immunofluorescencji lub testem ELISA.
W rozpoznaniu różnicowym należy uwzględnić m.in.: bakteryjne zapalenia jelit, zatrucia, obecność ciała obcego w przewodzie pokarmowym.
Podaje się antybiotyki aby zapobiec wtórnym nadkażeniom bakteryjnym. W ciężkich stanach kluczową rolę odgrywa niwelowanie skutków odwodnienia i zaburzeń elektrolitowych poprzez intensywne wlewy dożylne. Stosuje się środki przeciwbólowe, hamujące wymioty i biegunkę. Witaminy z grupy B i witaminę C. W dłużej przebiegającej chorobie konieczne jest odżywianie pozajelitowe. Można stosować leki stymulujące odporność. W okresie rekonwalescencji bardzo ważna jest odpowiednia dieta. Stosowanie surowicy w momencie rozwiniętego, objawowego zakażenia jest dyskusyjne.
Skuteczną metodą zapobiegania panleukopenii kotów jest szczepienie. Należy szczepić koty klinicznie zdrowe i odrobaczone. Szczepienia kociąt należy zacząć jak najwcześniej, zgodnie z zaleceniami lekarza weterynarii.
Wirus może przeżyć w temperaturze pokojowej nawet do roku i jest oporny na zamrażanie. Wszystkie przedmioty i powierzchnie, z którymi mógł mieć kontakt chory kot lub jego wydaliny, powinny zostać dokładnie odkażone (preparat Virkon). Z domowych środków można zastosować Domestos, ale musi on działać przez minimum 1 godzinę. Każdy, nawet dorosły kot przed wprowadzeniem do miejsca potencjalnie zagrożonego, powinien być zaszczepiony na 2 tygodnie przed tym zdarzeniem[2].
Coraz częściej weterynarze zalecają ozonowanie jako skuteczną metodę dezynfekcji w przypadkach eliminacji wirusów parwowirozy i panleukopenii. Często można spotkać się z tym, że lekarze polecają ozonowanie alergikom.[3]
Zakażenie FPV indukuje miejscową jak i ogólną odpowiedź immunologiczną. Długotrwałą odporność po przebytej infekcji warunkuje obecność przeciwciał w surowicy, których wysoki poziom w surowicy utrzymuje się jeszcze przez kilka lat. Kocięta, które przechorowały panleukopenię są odporne na ponowne zakażenie prawdopodobnie do końca życia.
Panleukopenia kotów (łac. Panleucopenia infectiosa felinum). Znana również jako: zakaźne zapalenie jelit kotów, tyfus koci, nosówka kotów (ang. Feline distemper), agranulocytoza.
Jest to zakaźna, wysoce zaraźliwa, wirusowa choroba wszystkich kotowatych. Przebiega z objawami zapalenia układu pokarmowego, wraz z towarzyszącą leukopenią. Choroba występuje na całym świecie.
Panleucopenia felina é uma doença viral que acontece aos gatos domésticos e outros membros das famílias Felidae, Mustelidae, Viverridae e Procyonidae. O agente causador é um vírus classificado como parvovírus felino [1]. Essa doença não é transmissível ao homem e nem a outros animais de estimação.
A Panleucopenia felina é uma moléstia altamente contagiosa caracterizada pelo aparecimento súbito dos seguintes sintomas:
Destaca-se ainda, que os sintomas podem variar entre diferentes organismos, podendo ocorrer de forma praticamente assintomática até os casos de morte súbita, sendo que nestes casos é comum que a panleucopenia seja confundida com envenenamento. No entanto a manifestação mais comum da doença é a depressão, falta de apetite, febre ao redor dos 40°C a 41,5°C, vómitos, desidratação, diarreia fétida, intensa e constante, além do gato apresentar o abdómen bastante sensível ao toque devido a presença de Gastroenterite.
O vírus da panleucopenia felina é um parvovírus pequeno pertencente à família Parvoviridae. Essa doença é considerada um dos distúrbios gastrointestinais mais mortais para os gatos, apresentando uma taxa de mortalidade de aproximadamente 80% dos indivíduos contaminados.
Esta doença tem sido registada em, praticamente, todo o planeta, podendo infetar os gatos domésticos e diversas espécies de felinos silvestres. O vírus infecta sobretudo gatos jovens, com idade inferior a 1 ano, sendo rara a ocorrência em espécimes adultos [1].
Os gatos que vivem soltos estão mais expostos à contaminação pelo vírus da doença. A vacinação é bastante eficaz, sendo que a incidência de panleucopenia é muito baixa nas populações de gatos que recebem as doses da vacina nos primeiros meses de idade. Contudo, foram relatadas ocorrencias da panleucopenia transmitida pelo colostro e pelo leite.
Devido à sua natureza altamente contagiosa, o vírus da panleucopenia felina dispersa-se por todo o ambiente onde se encontra um ser contaminado [1]. O reservatório do vírus são os próprios gatos, sendo que a transmissão é feita mediante as seguintes maneiras:
Animais doentes passam por um tratamento de elevada complexidade. Os animais que conseguem sobreviver mais de uma semana, evitando-se a todo o custo a desidratação, têm possibilidade plena de se salvar. No entanto, isso ocorre apenas em 20% dos casos, portanto a prevenção por meio da aplicação de vacinas ainda é a melhor forma de evitar a ocorrência do problema [2].
Após a cura, o vírus ainda continua a ser eliminado pela urina e fezes durante seis semanas, sendo portanto recomendado o isolamento do animal nesse período.
Panleucopenia felina é uma doença viral que acontece aos gatos domésticos e outros membros das famílias Felidae, Mustelidae, Viverridae e Procyonidae. O agente causador é um vírus classificado como parvovírus felino . Essa doença não é transmissível ao homem e nem a outros animais de estimação.
Panleukopénia alebo mačací mor je silne virulentné ochorenie s inkubačnou dobou 2–10 dní postihujúce len mačky. Ochorenie máva spravidla veľmi rýchly priebeh a môže pripomínať otravu. Prejavuje sa súčasnými prudkými hnačkami a zvracaním, veľmi často končí smrťou zvieraťa. Je možné proti nemu zviera zaočkovať.
Vírus napadá výstelku tráviaceho traktu, spôsobuje vredy a rozpad výstelkového epitelu. Zároveň sa znižuje množstvo bielych krviniek, čo zhoršuje imunitu zvieraťa. Nákazu si medzi sebou zvieratá väčšinou predávajú cez sekréty, ktorými sa kontaminuje napríklad voda na pitie. Postihnuté zviera je apatické, zvracia, má vodnatú hnačku, veľakrát je v stolici krv, neje a nepije. Pokiaľ je nákaza slabšia, je možnosť vyliečenia. Ak sa nakazí gravidná mačka, väčšinou potratí. Pokiaľ sa narodí živé mačatá, mávajú postihnutý mozoček, následkom čoho majú zhoršenú koordináciu pohybu alebo môžu byť aj slepé.
Tento článok je čiastočný alebo úplný preklad článku Panleukopénie na českej Wikipédii.
Panleukopénia alebo mačací mor je silne virulentné ochorenie s inkubačnou dobou 2–10 dní postihujúce len mačky. Ochorenie máva spravidla veľmi rýchly priebeh a môže pripomínať otravu. Prejavuje sa súčasnými prudkými hnačkami a zvracaním, veľmi často končí smrťou zvieraťa. Je možné proti nemu zviera zaočkovať.
Vírus napadá výstelku tráviaceho traktu, spôsobuje vredy a rozpad výstelkového epitelu. Zároveň sa znižuje množstvo bielych krviniek, čo zhoršuje imunitu zvieraťa. Nákazu si medzi sebou zvieratá väčšinou predávajú cez sekréty, ktorými sa kontaminuje napríklad voda na pitie. Postihnuté zviera je apatické, zvracia, má vodnatú hnačku, veľakrát je v stolici krv, neje a nepije. Pokiaľ je nákaza slabšia, je možnosť vyliečenia. Ak sa nakazí gravidná mačka, väčšinou potratí. Pokiaľ sa narodí živé mačatá, mávajú postihnutý mozoček, následkom čoho majú zhoršenú koordináciu pohybu alebo môžu byť aj slepé.
Kattpest orsakas av ett specifikt Parvovirus (FPV, feline (dvs katt-) parvovirus) som drabbar katter och även hundar (liknar hundens parvovirus). Viruset smittar från katt till katt men är motståndskraftigt och kan överleva länge i naturen. Sjukdomen ger symptom som kräkningar, diarréer och kan i svåra fall vara dödligt. Dräktiga smittade katter kan få missfall och avkomman kan födas med hjärnskador. Det finns vaccination för katterna i form av ett trippelvaccin som även ger immunitet mot calicivirus och herpesvirus.
Kattpest orsakas av ett specifikt Parvovirus (FPV, feline (dvs katt-) parvovirus) som drabbar katter och även hundar (liknar hundens parvovirus). Viruset smittar från katt till katt men är motståndskraftigt och kan överleva länge i naturen. Sjukdomen ger symptom som kräkningar, diarréer och kan i svåra fall vara dödligt. Dräktiga smittade katter kan få missfall och avkomman kan födas med hjärnskador. Det finns vaccination för katterna i form av ett trippelvaccin som även ger immunitet mot calicivirus och herpesvirus.
Virus panleukopenia feline (FPV), còn được gọi với nhiều cái tên khác nhau là viêm ruột truyền nhiễm mèo, viêm ruột Parvo ở mèo,[1] bệnh sốt ho ở mèo,[2] mất điều hòa, hoặc dịch hạch ở mèo,[3] là một tình trạng nhiễm virus ảnh hưởng đến mèo, bao gồm cả các loài mèo hoang và thuần hóa. Nguyên nhân là do virus Parvo ở mèo, họ hàng gần với cả hai loại Parvo ở chó, là một dạng bệnh Pravo loại 2 và bệnh viêm ruột chồn. Sau khi bắt đầu ký sinh, nó rất dễ lây và có thể gây tử vong cho mèo là vật chủ.[1] Một tên bệnh khác là Giảm bạch cầu ở mèo xuất phát từ số lượng tế bào máu trắng thấp (bạch cầu) được cho thấy bởi các động vật bị nhiễm bệnh.[3]
Giảm bạch cầu ở mèo đòi hỏi phải điều trị tích cực để mèo có thể sống sót, vì bệnh này có thể giết mèo trong vòng chưa đến 24 giờ. Điều trị bao gồm truyền máu toàn phần để cải thiện tình trạng giảm toàn thể huyết cầu, truyền dịch tĩnh mạch vì hầu hết mèo bị mất nước, tiêm kháng sinh A, B và C, IV để ngăn ngừa nhiễm khuẩn huyết, tình trạng tiến triển ở hầu hết mèo bị giảm bạch cầu nếu không sử dụng kháng sinh và nhập viện.
Một con mèo được chẩn đoán mắc FPV cần có biện pháp đầu tiên là cách ly, cô lập.[4]
Virus panleukopenia feline (FPV), còn được gọi với nhiều cái tên khác nhau là viêm ruột truyền nhiễm mèo, viêm ruột Parvo ở mèo, bệnh sốt ho ở mèo, mất điều hòa, hoặc dịch hạch ở mèo, là một tình trạng nhiễm virus ảnh hưởng đến mèo, bao gồm cả các loài mèo hoang và thuần hóa. Nguyên nhân là do virus Parvo ở mèo, họ hàng gần với cả hai loại Parvo ở chó, là một dạng bệnh Pravo loại 2 và bệnh viêm ruột chồn. Sau khi bắt đầu ký sinh, nó rất dễ lây và có thể gây tử vong cho mèo là vật chủ. Một tên bệnh khác là Giảm bạch cầu ở mèo xuất phát từ số lượng tế bào máu trắng thấp (bạch cầu) được cho thấy bởi các động vật bị nhiễm bệnh.
Панлейкопения («кошачья чумка») — вирусная болезнь кошек, отличающаяся высокой контагиозностью, характеризующаяся лихорадкой (высокая температура), поражением желудочно-кишечного тракта, респираторных органов, сердца, общей интоксикацией и обезвоживанием организма. Не опасна для человека.
Болезнь вызывается парвовирусом (лат. Virus panleukopenia feline) из группы парвовирусов размером 20-25 нм. Данный вирус имеет антигенное родство с возбудителями вирусного энтерита норок и парвовирусного энтерита собак. Геном вируса представлен однонитчатой молекулой ДНК. Смертность котят при данном заболевании составляет 90 %. Для людей вирус неопасен. Вирус поражает крипты тонкого кишечника, клетки костного мозга, лимфатическую систему.
Вирус парвовируса устойчив при pH 3,0-9,0, выдерживает нагревание до 60 °С в течение 1 ч, на него не действуют диэтиловый эфир, хлороформ, пепсин, трипсин. Срок жизни вируса во внешней среде достигает 1 года, в связи с чем он широко распространён в природе.
Основным источником заражения и распространителем инфекции служат больные и уже переболевшие панлейкопенией кошки, которые выделяют возбудителя болезни во внешнюю среду с фекальными и рвотными массами. Вирус в кале кошек появляется одновременно с первыми клиническими проявлениями панлейкопении, и ко второму-третьему дню от начала болезни его содержание в нём достигает максимума.
Существует мнение, что во время рвоты парвовирусы проникают также в верхние дыхательные пути кошки и далее выделяются во внешнюю среду воздушнo-капельным путём. Возможен также трансмиссивный механизм заражения — через кровососущих насекомых, в частности, блох. Также не исключается возможность внутриутробного заражения котят.
Болезни предшествует инкубационный период, который длится 3-10 дней. Затем появляются первые клинические проявления болезни, выраженность которых зависит от возраста животного, степени патогенности возбудителя, а также от иммунитета животного.
В случае острого протекания болезни состояние животного резко ухудшается, его температура быстро повышается до 41 °С и выше. Кошки отказываются от корма. Появляется рвота; рвотные массы имеют зеленоватo-жёлтый цвет, содержат слизь, иногда кровь. Моча кошек становится тёмнo-жёлтой с колебаниями до светлo-оранжевого. Фекалии становятся жидкими и со зловонным запахом, часто с примесью крови и фибрина. В них содержится значительное число вирусов. Слизистая оболочка ротовой полости кошек становится сухой и синюшной, к основной болезни также присоединяются конъюнктивиты и риниты.
Поведение заболевших животных резко изменяется: кошки прячутся в укромных местах, при лихорадке ищут прохладные места. Они лежат на животе, запрокинув голову и вытянув конечности, либо сидят сгорбившись в тёмном месте.
Кошки часто сидят над миской с водой, но не пьют её. Это связано как с обезвоживанием организма, так и с резко болезненными ощущениями в животе: кишечник растягивается жидкостью и газами, лимфоузлы брыжейки увеличиваются. Ввиду обезвоживания организма кошки сильно худеют, шерсть становится тусклой, кожа — сухой и вялой. У старых кошек может возникнуть отёк лёгких, сопровождающийся влажными хрипами. Кошки вне зависимости от возраста могут впасть в кому, у них могут возникнуть судороги.
В случае сверхострого течения панлейкопении происходит внезапная смерть кошки. При лёгком течении кошки испытывают только недомогание, а болезнь выявляется только при исследовании крови.
Острая форма заболевания протекает 1-10 дней. Болезнь весьма опасна: среди котят смертность достигает 90 %. Кошки, пережившие первые 3-4 дня, обычно выздоравливают, но остаются вирусоносителями.
Диагноз «панлейкопения» ставится на основании лабораторных данных: ПЦР или ИФА-диагностики крови либо фекалий. Заподозрить панлейкопению ветврачу позволяют результаты общеклинического анализа крови, в котором отмечается снижение количества лейкоцитов (лейкопения) до 4000, 3000 и ниже в 1 ммз. Наблюдается также сильное снижение количества нейтрофилов вплоть до полной нейтропении.
Лечение болезни является как симптоматическим, так и специфическим: животным капельно внутривенно и подкожно вводятся изотонические солевые растворы, глюкоза (5 %) 1-2 раза в день. Кроме того, кошкам вводятся спазмолитики, антибиотики (фармазин, цефтриаксон), кишечные абсорбенты (Энтеросгель, полисорб), обволакивающие (фосфалюгель, алмагель), пробиотики (лактобактерии), препараты, активирующие гемопоэз (витамин B12, железосодержащие препараты, фолиевая кислота), назначается специальная диета: лечебные смеси для энтерального питания (нутризоны). Препараты, усиливающие перистальтику (церукал, метоклопрамид) при диарее применять нельзя! Хорошие результаты дает использование филграстима и его аналогов. Применение иммуномодуляторов не имеет клинически доказанной эффективности. При необходимости делается переливание крови от здоровых и вакцинированных котов.
Чем раньше начато комплексное лечение, тем выше эффект. Лечение кошек, даже внешне выздоровевших, продолжают до полного завершения курса. У недолеченных кошек (неполное исчезновение всех симптомов, таких, например, как слабый аппетит) может произойти рецидив болезни.
Проводится ежегодно зарегистрированными поливалентными вакцинами.
Панлейкопения («кошачья чумка») — вирусная болезнь кошек, отличающаяся высокой контагиозностью, характеризующаяся лихорадкой (высокая температура), поражением желудочно-кишечного тракта, респираторных органов, сердца, общей интоксикацией и обезвоживанием организма. Не опасна для человека.
猫汎白血球減少症(ねこはんはっけっきゅうげんしょうしょう、英:en:feline panleukopenia|feline panleukopenia:FPL)とは猫汎白血球減少症ウイルス感染を原因とするネコ科動物の感染症。猫ジステンパー、猫伝染性腸炎とも表記される。猫汎白血球減少症ウイルス(別名「猫パルボウイルス」)はパルボウイルス科に属するDNAウイルスである。糞便を介しての感染および胎盤感染を引き起こす。妊娠動物の感染では流産や胎子の小脳形成不全を引き起こす。若齢動物は感受性が高い。症状は発熱、元気消失、脱水、嘔吐、下痢、血便などを示し、白血球の減少が認められる。病理学的所見として空腸や回腸の充出血、腸管リンパ節の腫大と出血が認められる。予防にはワクチンが使用される。治療にはインターフェロンの投与や、細菌の二次感染に対する抗生物質の投与を行う。
猫汎白血球減少症(ねこはんはっけっきゅうげんしょうしょう、英:en:feline panleukopenia|feline panleukopenia:FPL)とは猫汎白血球減少症ウイルス感染を原因とするネコ科動物の感染症。猫ジステンパー、猫伝染性腸炎とも表記される。猫汎白血球減少症ウイルス(別名「猫パルボウイルス」)はパルボウイルス科に属するDNAウイルスである。糞便を介しての感染および胎盤感染を引き起こす。妊娠動物の感染では流産や胎子の小脳形成不全を引き起こす。若齢動物は感受性が高い。症状は発熱、元気消失、脱水、嘔吐、下痢、血便などを示し、白血球の減少が認められる。病理学的所見として空腸や回腸の充出血、腸管リンパ節の腫大と出血が認められる。予防にはワクチンが使用される。治療にはインターフェロンの投与や、細菌の二次感染に対する抗生物質の投与を行う。
고양이 범백혈구 감소증(Feline panleukopenia, 범백혈구 감소증, 고양이 전염성 장염, 범백)은 고양이 파보 바이러스(Feline parvo virus, FPV)에 의해 발병하는 바이러스성 장염이다. 전염성이 매우 강하고 치사율이 높아 모든 고양이 종에게 치명적이다.[1] '범백혈구 감소증'이라는 이름은 이 질병에 감염된 동물들에게서 백혈구가 현저하게 감소하는 증상을 보이기 때문에 이러한 이름이 붙여졌다.[2]
주로 감염된 동물의 체액, 배설물 등의 접촉으로 감염되는데 이러한 매개체와 접촉하지 않았더라도 이 매개체와 접촉한 벼룩이나 빈대 등에 의해서도 감염될 수 있으며 감염된 동물과 접촉되었던 적이 있는 침구류, 음식 뿐만 아니라 착용했었던 의류나 신발에 의해서도 감염될 수 있다. 임상 징후는 일반적으로 노출 후 4 ~ 6 일 내에 발생하지만 2 ~ 14 일 내에 나타날 수 있으며 사람에게 전염되지는 않는다. 원인이 되는 FPV 바이러스는 구조적으로 매우 안정적이기 때문에 적절한 환경이 갖춰진다면 최대 1년까지 생존할 수 있다. 또한 이 질병에서 회복한 동물도 최대 6주까지 배설물 등에 바이러스가 남아있다.
FPV 바이러스는 숙주에 침입하여 가장 먼저 위장관 내벽을 공격하여 위장 전체적으로 궤양을 형성한다. 이로 인해 감염된 동물은 보통 혈변, 설사, 심한 탈수, 영양실조, 빈혈 등을 보이며 그 외 증상으로는 우울증, 무기력, 식욕 부진, 발열, 구토, 탈수로 인한 피부 탄력의 저하 등이 있고 또한 오랫동안 물을 마시기도 한다. 말기에는 저체온증도 보이며 패혈성 쇼크(septic shock) 및 파종성 혈관 내 응고(disseminated intravascular coagulation, DIC)까지 일으킬 수 있다. 대부분 이 질병에 감염된 고양이의 주된 사망 원인은 이차 감염 또는 설사로 인한 탈수가 대부분이다. 이차 감염은 FPV 바이러스에 감염된 고양이가 면역 체계에 문제가 생겨 이차 감염의 위험에 쉽게 노출되기 때문이다.[3]
임신 중인 고양이가 이 질병에 노출될 경우에는 FPV 바이러스가 뱃속 새끼에게 소뇌 형성 부전(cerebellar hypoplasia)을 일으킨다. 이 때문에 임신중인 고양이에게는 FPV 바이러스에 대한 예방접종을 하지않는다. 고양이의 FPV 바이러스는 개 파보 바이러스와 밀접하게 관련이 있고, 고양이에서 강아지로는 전염이 안된다.
일반적으로 고양이 범백혈구 감소증(FPL)의 임상적 진단은 특징적인 위장관 질환과 감수성 개체에서 심한 범백혈구감소에 기초하여 이루어지지만, 분변검사, 혈액배양등도 다른 질환을 배제하기 위해 일반적으로 실시된다. FPL의 감별진단으로는 살모넬라증, 장관 중독증, FIV, 고양이 백혈병감염증(FeLV), 크립토스포리디움증, 췌장염, 급성 내독소혈증을 동반한 패혈증, 톡소포자중, 복막염, 림프종 등을 포함한다. 예방접종이 되지않은 고양이에서, FPLV에 대한 항체는 이 질환을 가지고 있거나, 과거에 앓았음을 의미하지만, 많은 경우 무증상감염도 일어나기 때문에 항체검사는 신뢰하기 어렵다.
일반적인 고양이 범백혈구 감소증 백신으로 예방한다. 여러 다른질환과 혼합된 백신으로 이용가능하다. 이 백신의 여러질병에 대한 방어능력, 그리고 이 질환의 심각도가 크기 때문에 모든 고양이에서 추천된다. FPV는 매우 안정적인 바이러스이고, 매개체를 통해 감염이 이루어질 수 있어서 집안에만 사는 고양이에서도 감염될 수 있다.[4]
하지만 이 바이러스는 임신한 고양이의 새끼에게 소뇌 형성 부전이라는 치명적인 부작용을 일으키므로 임신중인 고양이에게는 예방 접종을 실시하지 않는다.
이 질병에 감염된 동물은 24시간 이내에 죽을 수 있을 정도로 치사율이 높기 때문에 발견 즉시 치료가 필요하다. 치료는 전체적으로 저하된 백혈구 수를 증가시키기 위해 전혈 수혈을 하기도 하고, 탈수에 의한 패혈증을 방지하기 위해서 항생제와 비타민A, B, C 등을 포함한 수액을 정맥 주사한다. 이 질병은 전염성이 매우 강하기 때문에 보통 동물병원에 입원시킨 후 격리치료를 실시한다.
FPV에 진단된 고양이는 첫째로 격리시켜야 한다.[5]
정맥투여로 탈수와 전해질을 교정하는 것이 중요하다. 감염된 고양이에서 장관 방어기전이 파괴되기 때문에, 세균이 혈액내로 침범할 수 있다. 패혈증을 막는것이 가장 중요하기 때문에, 광범위 항생제를 투여해야한다(정맥투여). 경구 식이급여가 유익한 효과를 보인다라는 연구가 있기 때문에 가능한한 오래동안 급이를 계속해야한다. 아주 소화흡수가 높은 식이를 급여해야하며, 구토가 지속된다면, 항구토제를 처방해야한다. 비타민보충제(특히 Vit.B)는 thiamine 결핍을 막기위해 투여한다. 식욕부진, 저단백혈증, 구토, 설사를 보이는 고양이에서 비경구영양공급이 필요하다.[6]
고양이 재조합 인터페론 오메가가 개에서 효과적이며, 세포배양내에서 FPV의 복제를 억제한다. 하지만 감염된 고양이에서는 아직 연구가 더 필요하며, 추후에 사용될 것으로 여겨진다.
생후 두 달 이내밖에 되지 않은 고양이는 약 95%의 높은 치사율을 보인다. 생 후 두 달이 넘은 고양이라 하더라도 치료를 실시함에도 불구하고 약 60~70%가 사망하며 치료하지 않을 경우에는 100% 사망한다. 성묘(성인 고양이)의 경우에는 치료를 실시했을 경우 치사율은 약 10~20%, 치료를 하지 않았을 경우 치사율은 약 85%를 보인다. 고령의 고양이는 신체적으로 노약해진 상황이므로 성묘보다 치사율이 다소 높다.
주로 발생하는 합병증으로는 거의 대부분 탈수 증상을 일으키며, 저나트륨혈증(Hyponatremia)과 전해질 불균형, 고열, 저체온증도 흔하게 발생한다. 또한 백혈구 수 감소로 인한 세균, 곰팡이, 다른 종의 바이러스에 이차 감염이 발생할 수 있다. 만약 파종성 혈관 내 응고가 발생할 경우 고양이에게 매우 치명적이다. 극단적인 상황으로 갈 경우 혈소판 감소증(thrombocytopenia)과 심각한 출혈성 합병증까지 일으킬 수 있다. 고양이들은 보통 이 질병을 이겨내 치료가 되면 대부분 모든 장기가 회복되어 생존하지만, 낮은 확률로 혈액이나 위장관에 후유증이 발생하였다는 사례가 보고가 된 적이 있으며, 드물기는 하지만 완치 후 후유증으로 심근증(Cardiomyopathy)이나 심근염(myocarditis)이 발생하였다는 보고도 있다.
고양이 범백혈구 감소증(Feline panleukopenia, 범백혈구 감소증, 고양이 전염성 장염, 범백)은 고양이 파보 바이러스(Feline parvo virus, FPV)에 의해 발병하는 바이러스성 장염이다. 전염성이 매우 강하고 치사율이 높아 모든 고양이 종에게 치명적이다. '범백혈구 감소증'이라는 이름은 이 질병에 감염된 동물들에게서 백혈구가 현저하게 감소하는 증상을 보이기 때문에 이러한 이름이 붙여졌다.