pt-BR
nomes no trilho de navegação


This description provides characteristics that may be relevant to fire ecology and is not meant for identification. Keys for identification are available (e.g., [69,73,115,152,153]).
Common velvetgrass is typically a pubescent, tufted, perennial grass. However, in the Carolinas and Atlantic Coastal regions, common velvetgrass behaves as an annual [37,115]. European studies revealed that life span and life history can vary with environmental conditions. Plants grown from seed collected from dry, southern European habitats flowered in their first year and died within 2 to 4 years. Plants grown from seed collected in northern Europe failed to flower in their first year and were longer lived. Plants grown from seed collected in exposed maritime habitats displayed a low, spreading growth form and produced leaves that were only 13 inches (32 cm) long, but plants from seed collected from continental habitats were erect and reached 28 to 35 inches (70-90 cm) tall [19].
Aboveground description: Common velvetgrass stems are generally erect, hollow, and grow to 12 to 39 inches (30-100 cm) tall [3,9,73,114,153]. At the base, stems may be somewhat prostrate and produce roots at the nodes [9,31,85]. Leaf blades are flat and measure 4 to 12 mm wide and 2 to 8 inches (5-20 cm) long [31,69,114,153]. Common velvetgrass produces a dense, compact panicle that can reach 6 inches (15 cm) long [3,114,153]. Spikelets are generally 2-flowered. Upper florets are staminate with fairly robust awns that become hooked when dry. Lower florets are perfect [149,153,164]. Common velvetgrass seeds measure 1.5 to 2.5 mm long [115,149].
Belowground description: The common velvetgrass root system is fibrous and concentrated at shallow depths. Boogie and others (1958, as cited in [134]) indicated that, while common velvetgrass roots may reach 35 inches (90 cm) deep, most roots occur in the top 4 inches (10 cm) of soil. In a heavily grazed pasture in Germany, 51% of common velvetgrass roots were in the top 4 inches (10 cm) of soil, and 16%, 18%, 11%, and 4% of the roots occurred in the subsequent 4-inch (10 cm) depth intervals (Klapp 1943, as cited in [14]).
Site conditions may affect root development. When widely spaced, common velvetgrass may produce "a dense network of fine, whitish, surface roots" on the ground beneath the shading of its own canopy. When water tables are high, root penetation is limited [14]. During a field experiment in the University of York experimental garden, shading reduced common velvetgrass root number and root dry biomass [42].
On Hartz Mountain in Germany, a researcher reported that common velvetgrass produced "subterraneous, elongated creeping rhizomes". Soils on this site had high metal concentrations. While this characteristic was not mentioned elsewhere in the literature, the researcher's examination of herbarium specimens revealed rhizomes on other European collections not associated with heavy metal concentrations (abstract in [40]).
Common velvetgrass is nonnative but widespread in North America. On the west and east coasts of North America and in Hawaii, common velvetgrass is a widespread nonnative species. Populations are less common in more inland states and provinces [70]. In southeastern Alaska, common velvetgrass was cultivated and had established outside cultivation by 1959 [3]. Common velvetgrass is common to abundant at low elevation sites in the Pacific Northwest [114,134]. In California, it occurs in all but the desert regions [125], and some have referred to it as a "new native" [67]. In Baja California, Nevada, and Arizona, common velvetgrass is likely restricted to northern habitats [79,80,141,165]. Common velvetgrass is essentially absent from the Great Plains [164] and may only occur in Missouri and eastern Kansas [57]. Scattered populations occur in Illinois [98]. In the northeastern United States and adjacent Nova Scotia, common velvetgrass is well established [53,134]. Populations are scattered in southern Quebec and Ontario [134] but common along the Atlantic Coast from North Carolina to Nova Scotia [37]. In Hawaii, common velvetgrass is widely distributed in all but the driest habitats [127] and is often found in pastures, wet disturbed areas, and on roadsides [153]. Grass Manual on the Web provides a distribution map of common velvetgrass in North America.
Common velvetgrass is native to Europe, western Asia, northwestern Africa, and the Canary Islands and is very common throughout temperate Europe [14,15]. A review reports that it was likely introduced several times to both the east and west coasts of North America as a contaminant or an intentional component of imported forage seed [113,134]. As of 1800, common velvetgrass occurred in many parts of North America [9]. Based on early North American floras, it occurred in Pennsylvania by 1755 and was frequent in 1814 [163]. In New England, common velvetgrass introductions probably occurred in the 17th century [92]. The first known collection of common velvetgrass from London, Ontario, occurred in 1879 [134]. In Hawaii, it was first collected in 1909 [153].
Potential for postfire establishment and spread: If common velvetgrass occurs in or around a burned area, it is possible in the postfire plant community. Studies described above suggest that common velvetgrass is often present and can be abundant in early postfire succession. This pattern does not appear to be affected by location, fire severity, fire frequency, fire season, or associated disturbances. Common velvetgrass abundance typically decreases with time since fire, although long-term studies are lacking. See FIRE ADAPTATIONS AND PLANT RESPONSE TO FIRE for details.
Common velvetgrass establishes on burned sites from off-site or seed bank sources, and may sprout from surviving basal plant parts. Common velvetgrass seeds often occur in the soil even when mature plants are absent or occur in low abundance on the site. Seeds are readily dispersed by a number of vectors (see Seed dispersal). Germination from the seed bank on burned sites is possible, since germination was not affected by up to 10 minutes of exposure to temperatures of 180 to 230 °F (80-110 °C) [120]. Buried seed may also survive on severely burned sites. A review reports that viable common velvetgrass seed was collected from a maximum depth of 20 inches (50 cm) [136]. Anecdotal accounts from Hawaii [127] and Argentina [51] suggest that common velvetgrass may sprout following top-kill. Postfire sprouting was not reported in the majority of reviewed literature.
Minimizing soil disturbances and maintaining high cover of native plants may help prevent or minimize common velvetgrass establishment and spread after fire. For more detailed information on preventing postfire establishment and spread of invasive species, see the following publications: [6,22,55,147].
Use of prescribed fire as a control agent: Fire is not likely useful in the control of common velvetgrass because becaue it is likely to establish, persist, and/or spread after fire (see FIRE ADAPTATIONS AND PLANT RESPONSE TO FIRE). Cover of common velvetgrass typically exceeds that of native Hawaiian grasses after fire, so prescribed fire is not recommended in common velvetgrass habitats in Hawaii [128]. Integrating mowing or grazing and prescribed fire treatments, however, may decrease common velvetgrass dominance [113].
FIRE REGIMES in the native range of common velvetgrass were not described in the reviewed literature. Given that common velvetgrass persists on sites in Washington that have burned almost annually for the last 50 years [145,146], frequent fire is likely tolerated. Lack of fire is more likely to reduce the abundance and/or persistence of common velvetgrass than frequent fire.
While common velvetgrass could potentially increase fine fuel loads in many of its nonnative US habitats, this is described only in Hawaii and California. Establishment of common velvetgrass may reduce the frequency and/or size of gaps in subalpine vegetation and increase fire potential through increased fuel continuity [128]. In coastal grasslands of Sonoma, California, litter accumulations are often greater in common velvetgrass communities than in annual grasslands [13], which may affect fire probability and/or behavior. This topic is also addressed in Litter accumulation. The Fire Regime Table provides fire regime information for many vegetation types and plant communities in which common velvetgrass may occur. Find further fire regime information for the plant communities in which this species may occur by entering the species name in the FEIS home page under "Find FIRE REGIMES".
Greenhouse and field studies indicate that common velvetgrass germinates best in full light conditions and fluctuating temperatures. Low temperatures, dry conditions, and burial can decrease or delay germination. In a review, Beddows [14] reports that common velvetgrass germinates "readily". In the laboratory, 100% of common velvetgrass seeds collected from an encina (Quercus ilex subsp. rotundifolia) woodland in central-western Spain germinated [112]. Germination rates of common velvetgrass seeds collected from a permanent pasture in England ranged from 90% to 99% after up to 48 weeks of dry storage [167].
Light requirements: While full light and exposed conditions are typically best for common velvetgrass germination, some germination also occurs when seeds are buried and/or in the dark. Large temperature fluctuations may increase germination in dark conditions. At nearly constant temperatures of 54 °F (12 °C) and 75 °F (24 °C), common velvetgrass germination percentages in the dark were 48% and 30%, respectively [44]. After conducting numerous laboratory studies on common velvetgrass seed germination, Thompson and others [135] found that common velvetgrass germination was greater than 75% when temperatures fluctuated from 0 to 22 °F (0-12 °C). Emergence rates and germination percentages of common velvetgrass seed in the greenhouse were not different between shading levels of 0%, 33%, 53%, and 78% [64].
Depth of burial: Increasing depths of burial beneath soil or litter typically decrease common velvetgrass germination. In the greenhouse, maximum common velvetgrass germination was 89% under 0.4 inch (1 cm) of soil, 75% under 1.2 inches (3 cm) of soil, and 26% under 2 inches (5 cm) of soil [167]. In cleared coastal prairie plots in northern Marin County, California, common velvetgrass germinated at nearly 90% on sites without litter. Under 0.4 inch (1 cm) of litter, germination decreased to about 50% but was still significantly greater than that of 4 native prairie grasses (P-value not reported). Germination of common velvetgrass was about 30% under 1.2 inches (3 cm) of litter, which was not different than germination of the native species [117]. This study is also discussed in Litter accumulation.
Temperature, moisture: Cold temperatures and dry conditions can reduce or delay common velvetgrass germination. Common velvetgrass seed collected from a California coastal prairie had the lowest germination (nearly 30%) when growth chamber temperature fluctuatations were slight, between 37 and 48 °F (3-9 °C). At higher and greater fluctuations of temperatures (46 to 68 °F (8-20 °C)), germination was near or just below 50% [117]. In the greenhouse, common velvetgrass seeds from a grassland in Oxford, England, germinated best when temperatures alternated below and above 68 °F (20 °C). Germination percentages were lowest at a constant temperature of 68 °F (20 °C) [166]. Diurnal temperature requirements may function as a "gap-detecting mechanism", allowing common velvetgrass seeds to sense gaps in the canopy through temperature changes (Thompson and others 1977, as cited in [166]). When common velvetgrass was exposed to high temperatures of 180 to 230 °F (80-110 °C) for up to 10 minutes, germination was not affected. Germination was inhibited after 10 minutes at 300 °F (150 °C), although seeds were not "destroyed". Smoke exposure did not affect germination [120].
Common velvetgrass emergence was affected by sowing date, which was related to temperature and moisture field conditions in Oxford, England. Generally seedlings emerged 1 to 2 weeks after sowing. When temperatures were low or conditions were dry, emergence was delayed. Emergence was evaluated in each month of the year and was lowest for seeds sown from April to July. Mild winter and hot, dry summer conditions prevailed during this experiment [156]. In nonirrigated western Oregon pastures, common velvetgrass typically germinates with fall rains. In a field study, emergence was delayed when fall moisture was low [64].
In North America, common velvetgrass habitats include pastures, cultivated fields, meadows, ditch banks, lawns, roadsides, and other disturbed sites [9,31,73,79,114,153].
Climate: A review reports that common velvetgrass tolerates a wide range of moisture conditions within temperate climate regimes. While common velvetgrass grows best in moist areas, it grows well in very wet conditions and tolerates "moderate" periods of drought [134]. The northern limit for common velvetgrass is near the January isotherm of 28.4 °F (-2 °C). The 80 °F (26.7 °C) July isotherm approximates common velvetgrass' southern boundary in Europe and the Mediterranean. Beyond this southern boundary, precipitation from May to October is typically less than 5 inches (130 mm) and is likely the reason for common velvetgrass' absence [14].
Climates are similar in common velvetgrass' nonnative North American habitats. In British Columbia, common velvetgrass is common in temperate cool semiarid and mesothermal climates. Evaporation exceeds transpiration and the average annual temperature is less than 64 °F (18 °C) in semiarid habitats. In mesothermal habitats the average temperature in the warmest month is less than 73 °F (23 °C) and fewer than 4 months see temperatures below 39 °F (4 °C) [83]. Common velvetgrass is common in the Willamette Valley, where winters are cool and wet, and summers are warm and dry. Temperatures in January and July average 46 °F (8 °C) and 82 °F (27.5 °C), respectively. Based on 30 years of records, annual precipitation averages 43 inches (1,100 mm) [126]. In Pacific coastal areas, environmental conditions are more harsh and include wind, salt spray, and fog [8]. On the Bodega Marine Reserve, in Sonoma California, annual precipitation, most of which occurs from November through March, averages 34 inches (860 mm). Frequent fog moderates drought conditions [13], and common velvetgrass utilizes fog as a water source [29]. Humid climates prevail in the montane rain forest zone in Hawaii Volcanoes National Park, where common velvetgrass is common. Annual precipitation averages 98 inches (2,500 mm) at high elevations and 59 inches (1,500 mm) at low elevations [4].
Climate change: Common velvetgrass may experience increased growth with elevated CO2 levels. In a controlled study, common velvetgrass monocultures grown in elevated CO2 produced significantly more biomass than when grown in ambient conditions (P<0.001). After 2 months at elevated CO2 levels, aboveground biomass of common velvetgrass increased by 44% and belowground biomass increased by 135%. Researchers also noted changes in nitrogen cycling, which, depending on native species responses to elevated CO2, could affect competitive outcomes in mixed communities [10,11]. Increases in common velvetgrass biomass were also noted by Jongen and Jones [77]. When common velvetgrass was grown with 3 other grasses at elevated CO2 levels, increases in common velvetgrass biomass exceeded those of the other grasses. Common velvetgrass tillering increased by 25% with elevated CO2.
Elevation: Throughout North America, common velvetgrass occurs from sea level to 7,500 feet (2,300 m) [9]. In British Columbia, occurrence of common velvetgrass decreases with increasing elevation [83].
Common velvetgrass elevation range by state State Elevational range (feet) Arizona 4,500-7,000 [80] California below 7,500 [69,101] Hawaii 2,500-10,700 [153] Nevada 3,500-6,800 [79] northern New Mexico 5,000-6,000* [93] Utah 4,990 [164] *As of 1981, common velvetgrass was not described in New Mexico; this is an expected distribution.Soils: Although common velvetgrass tolerates a wide range of soil conditions [9], it is often described as occurring on moist sites [125,149]. In British Columbia, common velvetgrass is most common on fresh to very moist soils [83]. In California, common velvetgrass is found in all but the desert regions and grows best in moist, rich soils [125]. In the coastal prairies of California, common velvetgrass is rare on hilltop or steep sites that dry out early in the season (Thomsen, personal observation, cited in [140]). In the eastern United States, common velvetgrass often occurs on damp, moist, or poorly drained sites [149]. In controlled studies, as the water table height increased, common velvetgrass growth decreased. However, on sites with elevated water tables, common velvetgrass developed fine roots at the soil surface and adventitious roots at the plant base, suggesting a possible long-term adaptation to a high water table [160].
Native habitats: A review reports that although common velvetgrass may be absent from shallow soils in areas that experience severe drought, a wide range of soil types is tolerated. Coastal areas that receive salt-spray are occupied by common velvetgrass [14]. In waterlogged soils, growth of common velvetgrass is often reduced [44]. Near Sheffield, England, common velvetgrass grew on soils with pH levels of 3.5 to 8 but was most abundant where the pH was 5 to 6 (Grime and Hodgson, personal communication, cited in [157]).
Nonnative habitats: In North America, soils vary in common velvetgrass habitats. In one review, common velvetgrass is reportedly frequent on poor, moist soils [113]. In a review from Canada, common velvetgrass is considered most common in infertile grasslands [134]. In British Columbia, common velvetgrass often occurred on nitrogen-medium and exposed mineral soils [83]. Sandy or gravelly soils were preferred habitat in Baja California [165]. After analyzing the vegetation and environmental data for 184 plots in Oregon white oak savannas in the Cowichan Valley of southeastern Vancouver Island, researchers found that common velvetgrass occurred most often on sites with shallow soils, 4 to 8 inches (10-20 cm) deep [91]. The common velvetgrass-sweet vernalgrass community type in Oregon was most common on deep soils with thick litter layers that averaged 2.6 inches (6.5 cm) deep. Soil pH averaged 5.7, and soil depth averaged 57 inches (146 cm). Soils averaged 13% clay, 31% silt, 56% sand, and 15.5% organic matter [119].
In many of its nonnative habitats, common velvetgrass is not described as a serious weed; however, many studies indicate that common velvetgrass' allelopathic potential, rapid early development, litter accumulation, response to disturbances, and nutrient additions can negatively impact associated native vegetation.
Impacts: Common velvetgrass is referred to as a problematic species in Hawaii but is often considered a species of lesser concern in other parts of its nonnative North American range. In reviews from the Hawaiian Islands, common velvetgrass is considered "disruptive" and described as "forming dense stands that appear to inhibit recruitment of natives" [34]. Establishment on disturbed sites in Hawaii is often rapid [127]. Invasiveness ratings of common velvetgrass are likely relative; when associated with more invasive weed species, it is less likely to be described as a problem.
Throughout most of its nonnative range, common velvetgrass is either not listed on invasive plant lists [56,94] or is referred to as a "minor weed" [134], not a "major problem species" [113], moderately invasive [151], or "not readily invading natural areas" [82] as of this writing (2009). Although prevalent in Oregon and Washington, common velvetgrass is absent from many invasive species lists for the area [56]. Common velvetgrass is not listed in the Invasive Plant Atlas of New England [94], although it is well established in the area. Potentially problematic common velvetgrass growth characteristics are discussed below; these may result in impacts that affect a local scale in the nonnative range.
Allelopathy: In laboratory tests, common velvetgrass showed possible allelopathic properties. When common velvetgrass and garden sorrel (Rumex acetosa) seedlings were grown in sand that was collected beneath a common velvetgrass monoculture, growth of both species was "markedly depressed" as compared to controls, even when nutrients were added (Al-Mashhadani and Grime, personal communication, cited in [158]). Germination and radicle extension were significantly lower for bulbous canarygrass (Phalaris aquatica) and orchardgrass (Dactylis glomerata) seeds kept moist with water containing common velvetgrass leaf extracts than for those kept moist with deionized water (P=0.001) [154].
Rapid early growth: Rapid germination and seedling growth may allow common velvetgrass establishment and spread in a variety of habitats. In a greenhouse experiment, the maximum relative growth rate of common velvetgrass was 2.01/week. Soon after seedling establishment, 4 weekly harvests were made to calculate this growth rate, which was high compared to other species evaluated [59]. In another greenhouse experiment, growth rates of common velvetgrass were 42 mg and 65 mg/g/day in low- and high-nitrogen environments, respectively. Growth rates were calculated from 10 weekly harvests [20].
Early germination and rapid seedling growth may allow for the development of stable common velvetgrass stands that limit the growth of associated species. Fifty days after seeding, common velvetgrass seedlings produced the greatest dry weights of 6 western Oregon pasture species grown in the greenhouse [64]. When seeds collected from coastal prairie in northern Marin County, California, were monitored in growth chambers, common velvetgrass germinated more rapidly than red fescue. However, the final germination rate of red fescue was 60% higher than common velvetgrass [117]. Common velvetgrass biomass was significantly greater than that of Hawaii's alpine hairgrass after 6 months of growth in low-light/low-nutrient, high-light/low-nutrient, and low-light/high-nutrient treatments (P<0.05). In high-light/high-nutrient conditions, alpine hairgrass biomass was greater than that of common velvetgrass, but not significantly. Common velvetgrass allocated more biomass to roots than did alpine hairgrass [50].
In greenhouse and field experiments, common velvetgrass was most abundant, had the highest growth rate, and, as a seedling, was the most resistant to invasion when compared to other British Columbia pasture species. In the greenhouse, common velvetgrass was most abundant in patches established from seed. Seedling patches resisted invasion most. Based on comparisons made with common velvetgrass tillers collected from older pastures, researchers characterized common velvetgrass as an "r-type" species that likely requires repeated colonization opportunities to maintain a viable population in pastures [143]. In patchy coastal prairie vegetation in California, patches of common velvetgrass inhibited establishment of other species when seed was introduced. Although some seedlings emerged in common velvetgrass patches, none of these produced seed within 2 years of establishment. In patches of nonnative annual grasses, common velvetgrass establishment was successful. An input of 12,442 common velvetgrass seeds/0.25 m² produced 39.2 common velvetgrass seedlings/0.25 m². Newly established individuals in annual grass patches produced up to 21 seeds/0.25 m² and 5.1 seedlings/0.25 m². Common velvetgrass also had some establishment in perennial grass patches, but none of these plants produced seed within 2 years [108].
A common velvetgrass monoculture established in the field near Bristol in the United Kingdom severely restricted the growth of European white birch (Betula pendula) seedlings. One year following planting, the diameter of European white birch seedlings averaged 2.8 mm when grown with common velvetgrass and 8.4 mm in the absence of common velvetgrass. Seedling heights averaged 8.7 inches (22.2 cm) with and 24.7 inches (62.7 cm) without common velvetgrass [168].
Litter accumulation: In California grasslands, high productivity and reduced litter decomposition in common velvetgrass grasslands may affect regeneration potential and species composition. On the Bodega Marine Reserve in Sonoma County, California, common velvetgrass stands (aboveground biomass 836 g/m²) were more productive than annual grassland stands (aboveground biomass 534 g/m²). Standing litter accumulations in common velvetgrass stands were 1,537 g/m² and in annual grasslands were 766 g/m². From exclusion experiments, researchers learned that the dominant detritivore in the area, Porcellio scaber, did not consume common velvetgrass litter. Increased litter in common velvetgrass stands could affect seedling recruitment as well as fuel loads, fire potential, and fire behavior [13]. In field studies in coastal prairie in northern Marin County, California, common velvetgrass litter decreased germination of native grasses more than that of common velvetgrass. Under 0.4 inch (1 cm) of litter, common velvetgrass germination was about 50% lower than germination on bare soil; however, germination of common velvetgrass was still significantly greater than that of Pacific hairgrass, red fescue, Pacific reedgrass (Calamagrostis nutkaensis), and purple needlegrass (Nassella pulchra) (P-value not reported). Under 1.2 inches (3 cm) of litter, common velvetgrass and native grass germination were not different [117].
Control: While several methods may be useful to control common velvetgrass, it is likely that severe defoliation and repeated treatments may provide the best control. Evaluation of associated vegetation and potential increases in these species may affect management decisions. In a greenhouse study using monocultures of 6 grasses and 4 legumes, researchers found that introduced thistle seed (Carduus nutans and Cirsium vulgare) emergence was lowest in common velvetgrass stands [155]. Management decisions in common velvetgrass' nonnative habitats may involve making value judgments between nonnative species.
Some researchers suggest that marking common velvetgrass treatment areas in the early morning when dew is trapped in its velvety hairs may help to focus control efforts and minimize nontarget effects [46].
Photo taken in Maui, HI,Flooding/salinity: Common velvetgrass experienced high mortality when partial dike removal occurred in a 15-year-old pasture on the Salmon River Estuary in Lincoln County, Oregon. Cover of common velvetgrass was up to 70% in the pasture before dike removal. In the 1st growing season after dike breaching, common velvetgrass suffered high mortality and averaged less than 5% cover. By the 2nd growing season, common velvetgrass was essentially absent. Before dike removal, salinity in the pasture was 0 to 3 ppt and after breaching was 11 to 39 ppt [97].
Fire: For information on the use of prescribed fire to control this species see Fire Management Considerations.
Prevention: Methods that may limit the establishment of common velvetgrass in lawn or pasture plantings are discussed by Fitzsimmons and Burrill [46].
Physical and/or mechanical: Some suggest that hand-pulling and hoeing, while labor intensive, can decrease common velvetgrass abundance [113]. A review reports that intense grazing or mowing may limit common velvetgrass establishment and spread [161]. Mechanical methods to control common velvetgrass are described by Fitzsimmons and Burrill [46].
It is important to note that mowing equipment has the potential to disperse common velvetgrass seed. After mowing in a common velvetgrass-dominated grassland in the Netherlands, 86% of the seeds removed from the mower were common velvetgrass seeds [132].
During controlled studies conducted outdoors and in a greenhouse, short cutting heights and increased cutting frequencies decreased common velvetgrass yield. When plants were cut between mid-March and early May, regrowth produced panicles by July 8, but when cut in early June, no panicles were produced on regrowth [159].
In the Willamette Valley, mowing and cutting led to increased common velvetgrass inflorescence production. Plants were mowed short, cut to the base twice, or burned twice in the fall. Mowing and cutting increased the reproductive potential of common velvetgrass. Fire generally decreased inflorescence production; details are discussed in the Western United States section of Fire Ecology [26]:
Changes in the number of common velvetgrass inflorescences/plant between pretreatment and first growing season after 1st and 2nd treatments [26] Treatment Mowed to 8-12 cm heights Cut at base Fall fire Control Difference between pretreatment and 1st year after single treatment +4.45 +1.34 -0.7 -0.38 Difference between pretreatment and 1st year after 2 treatments +11.50 +5.46 -0.7 -0.80Biological: No information is available on this topic.
Chemical: Herbicides potentially useful in common velvetgrass control are discussed in the following reviews: [46,134,161]. McHenry (1985, cited in [113]) suggests herbicide treatments may be most effective in the spring or when the first seed head appears because translocation to the roots is likely at that time.
Integrated management: A review suggests that mowing or grazing combined with prescribed fire treatments may decrease common velvetgrass dominance [113].
In the western United States, common velvetgrass is consumed by game birds, deer, and elk. According to Blakely and others [18], common velvetgrass is a "key" food for California quail (Callipepla californica). Common velvetgrass occurred in more than 15% of 222 sampled crops. In a 20-year-old burned area in northwestern Oregon's Tillamook region, a researcher noted heavy grazing of common velvetgrass, although quantitative measurements were not made [32]. In the Mount St Helens Blast zone, common velvetgrass was predominant in fall elk diets. The average density of common velvetgrass in summer-collected elk feces ranged from 1.2% to 2% and ranged from 2.2% to 5.1% for fall-collected feces [95,96]. In coastal prairie and coastal scrub vegetation in California's Point Reyes Peninsula, common velvetgrass made up 15% to 41% of elk diets from August to December in the second year of a fecal analysis study. In the first year of study, common velvetgrass was less prevalent and made up a high of 9% in April diets. Common velvetgrass made up only a trace of deer diets in any year or season in the study area [54]. On California's Tomales Point Elk Reserve, elk grazing reduced the abundance of common velvetgrass in open grasslands [75].
Palatability/nutritional value: Although consumed by elk and deer, common velvetgrass is not considered very palatable [70]. Common velvetgrass has been described as "without forage value" [85] and "not well liked by stock" [131]. Watt [157] reports that common velvetgrass is considered palatable early in the growing season, but palatability decreases as plants reach the flowering stage.
Digestibility and nutrient content of common velvetgrass in western US habitats are provided in the following references: [96,118]. Digestibility and nitrogen were greatest in the vegetative stage in the Mount St Helens blast zone [96].
In both its native and nonnative ranges, common velvetgrass occupies a wide
range of habitats. In Europe common velvetgrass occurs in pastures, grasslands,
wet to mesic meadows, and open forests and woodlands [14,161].
Pacific Coast: Most of the
information about North American common velvetgrass populations comes from the
Pacific Coast states, British Columbia, and Hawaii. In the Pacific
Coast states, common velvetgrass occurs in the north coastal shrub cover type
that is discontinuous from Washington's Olympic Peninsula to Santa Cruz,
California, and in the coastal prairie cover type that occurs from Oregon to
Monterey, California [8,12,67,107]. In Washington's Puget Trough,
common velvetgrass is a typical understory species in Douglas-fir–Pacific
madrone/pink honeysuckle (Pseudotsuga menziesii-Arbutus menziesii
/Lonicera hispidula) forests if grazed or near a disturbed or developed
area [25]. In the Oregon Coast ranges, common velvetgrass is often dominant in
the understory of red alder (Alnus rubra) stands before the understory
becomes shrub dominated (Henderson 1970, as cited in [49]). In the Willamette
Valley, common velvetgrass is frequent in Oregon white oak (Quercus garryana)
woodlands and tufted hairgrass (Deschampsia caespitosa) grasslands
(review by [49]).
Common velvetgrass is recognized in the following vegetation classifications:
Hawaii: Common velvetgrass is often
associated with grazed areas or sites with feral pig activity. The following vegetation
types are potential common velvetgrass habitat in Hawaii: koa-māmane
(Acacia koa-Sophora chrysophylla) forests, koa-`ōhi`a
(Metrosideros polymorpha) montane mesic forests, `ōhi`a montane wet
forests, Hawaii blackberry (Rubus hawaiensis) shrublands, pūkiawe-`ōhelo `ai
(Styphelia tameiameiae/Vaccinium reticulatum) shrublands, and alpine
hairgrass (Deschampsia nubigena) grasslands [153].
Atlantic Coast: In Massachusetts,
common velvetgrass occurs in little bluestem (Schizachyrium scoparium)
and "weedy" sandplain grasslands [39]. In West Virginia, it is
common in maintained hay meadows [48]. In the southern Appalachians
of North Carolina, common velvetgrass cover was 17% in old fields
dominated by common cinquefoil (Potentilla simplex) [27].
Studies from native and nonnative ranges indicate that common velvetgrass produces an abundant seed bank that is important to population persistence. A review of studies conducted in northwestern Europe reported that common velvetgrass seed may remain viable in the soil for more than 12 years. In one study, a maximum number of 16,900 common velvetgrass seeds/m² occurred in a sample of the top 2 inches (5 cm) of soil from a natural habitat. Another study reported collecting viable common velvetgrass seeds from a depth of 20 inches (50 cm) [136]. In other reviews, 5% of common velvetgrass seed reportedly germinated after 12 years of storage in a laboratory [14], and at the Welsh Plant Breeding Station, 14% of common velvetgrass seeds germinated after 10 years of burial beneath 5 inches (125 mm) of mineral soil [157].
Native habitats: Common velvetgrass seed banks can be extensive in disturbed and undisturbed habitats. A review reports that 70,000 common velvetgrass seedlings/acre emerged from the top 7 inches (20 cm) of soil collected in an undisturbed bentgrass-fescue (Agrostis-Festuca spp.) grassland in Kerry Hills, United Kingdom. No seedlings emerged from soil samples collected at depths below 7 inches (20 cm) [14]. When seed banks from 38 western European sites were compared, common velvetgrass was most common in the "extensively managed" grasslands [16]. Field and greenhouse studies conducted in Norfolk, United Kingdom, showed that common velvetgrass colonized artificially created gaps from soil-stored seed. Although common velvetgrass cover was less than 0.3% in the field, an average of 150 common velvetgrass seedlings/m² emerged from soil samples taken to 2-inch (5 cm) depths. Three common velvetgrass seedlings/m² emerged from soil samples collected from 12- to 14-inch (30-35 cm) depths. When gaps were created, common velvetgrass establishment was lowest on sites where vegetation was removed and soil was inverted to a depth of 14 inches (35 cm) [103].
Nonnative habitats: There is little evidence that seed bank dynamics are different between native and nonnative common velvetgrass habitats. If common velvetgrass is present in the aboveground vegetation, banked seed is nearly certain. On the University of British Columbia research forest, common velvetgrass seed was not recovered from seed traps or soil in old-growth mixed-conifer forests. In recent clearcuts, however, the frequency of common velvetgrass was 21.9% in the aboveground vegetation, 6.3% in seed trap collections, and 3.1% in the seed bank [81]. Although clipping reduced common velvetgrass seed set by 97% in a coastal prairie in Van Damme State Park, California, common velvetgrass seedlings emerged at a high rate, suggesting germination of soil-stored seed. On clipped plots, about 29 common velvetgrass seedlings/m² emerged, and on unclipped plots about 5 common velvetgrass seedlings/m² emerged [17]. It is possible that removal of aboveground vegetation improved germination and establishment conditions on clipped sites. In coastal prairies of Sonoma County, California, common velvetgrass emergence occurred with and without seed rain. On plots where seed rain was allowed, 1.8 common velvetgrass seedlings/m² emerged from soil collected in a common velvetgrass-dominated patch type and 12.0 seedlings/m² emerged from a Pacific hairgrass (Deschampsia holciformis)-dominated patch type. In the Pacific hairgrass patch, cover of common velvetgrass was almost 85% lower than in the common velvetgrass patch. When seed rain was excluded, 2.6 and 3.9 seedlings/m² emerged from soils collected in the common velvetgrass patch and the Pacific hairgrass patch, respectively. The researcher suggested that common velvetgrass may be invading Pacific hairgrass patches as succession proceeds [107].
There are many potential common velvetgrass seed dispersal vectors. Seeds are easily shed [14], and a large spikelet surface area encourages wind dispersal [14,134]. In a field study in northern California coastal grasslands, 90% of common velvetgrass seeds dispersed within a 17-foot (5.2 m) radial distance from the parent plant. Half of all seeds fell within a 5.6-foot (1.7 m) distance [110]. Water dispersal may be possible, and human and other animal vectors likely aid in seed dispersal.
Water: Buoyancy of comon velvetgrass seed suggests that it may be dispersed by water. In stagnant water, 54% of common velvetgrass seeds remained floating after 25 days and 9% after 90 days. In moving water, 47% remained floating after 25 days and 2% after 90 days. Germination of floating seeds was not tested [150].
Human activities: Mowing equipment was likely an important dispersal vector for common velvetgrass in the Netherlands. After mowing in common velvetgrass-dominated grasslands, 86% of seeds removed from equipment were common velvetgrass [132].
Animals: Worms, birds, rabbits, and cattle are possible dispersers of common velvetgrass seed. McRill (1974, as cited in [121]) reported that common velvetgrass seed was a major component of earthworm castings collected from grasslands in North Wales. Worms are likely important in the burial and unearthing of common velvetgrass seed (McRill 1974, as cited in [156]). Not all birds are likely to disperse common velvetgrass seed. Common velvetgrass seeds that passed through the digestive tract of sparrows were killed, but seeds passing through the digestive tract of rooks had only slightly reduced viability (Krach 1959, as cited in [157]). A small number of common velvetgrass seedlings emerged from rabbit pellets collected from an acidic grassland in Norfolk, United Kingdom. Although field and greenhouse studies indicated that the seed bank was most important to the colonization of bare patches, dispersal and establishment from rabbit pellets was possible [103]. For more on this study, see Seed banking. Common velvetgrass also germinated from cattle dung collected from heather (Calluna spp.) moorland in northeastern Scotland [162].
Predation: In a coastal prairie in Sonoma County, California, predation of common velvetgrass seed was low. When petri dishes of common velvetgrass seed were left out for 3 weeks in an annual grassland, only 6% were removed [107].
A review reports that although seed is generally only produced by lower florets, common velvetgrass is "notoriously a prolific seed producer".
Studies conducted in native and nonnative habitats indicate that common velvetgrass seed production can vary by vegetation type and sowing date. In Britain, common velvetgrass produced 63 to 611 spikelets/panicle in a greenhouse setting [15]. In a "closed" common velvetgrass-dominated grassland in Bangor, United Kingdom, the average number of common velvetgrass seeds/panicle was 270 but ranged from 100 to 380. Average seed production was 19,000 seeds/m² (Mortimer 1974, as cited in [157]). In coastal prairies of Sonoma County, California, common velvetgrass seed rain was 82,300 seeds/m² in a patch type where 91% of the relative cover was common velvetgrass. Seed rain was less than 6,000 seeds/m² in a patch where the relative cover of common velvetgrass was 4.6% [107]. For more on common velvetgrass seed production on newly colonized sites, see Impacts.
During field experiments in Oxford, England, sowing date affected common velvetgrass panicle and seed production. Plants from seed sown in November or December failed to flower. Plants from seed sown from January to June produced large numbers of panicles and seeds/plant. Panicle/plant production decreased from 759 on plants from June-sown seed to 184 on plants from July-sown seed. The greatest number of seeds produced per plant was 240,000 on plants from March-sown seed. The summer was very hot and dry and the winter very mild during these field experiments [156].
Seeds are generally viable soon after they are produced. When researchers tested seed germination at increasing time since anthesis, they found viable seeds 9 days after seed shed. Twenty days after anthesis, germination was 100% [14]. Another review notes that successful flowering depends on vernalization, and that longer cold periods often translate into a longer flowering period [134].
In its nonnative range, common velvetgrass is known as a rapid growing seedling. Late-fall sowing dates and shading can decrease seedling growth and survival, but spring moisture can increase seedling establishment and survival. While common velvetgrass establishment is often associated with disturbances in both its native and nonnative ranges [7,45,102,142], the bulk of information about seedling establishment and survival on disturbed sites comes from native habitats.
Seedling growth: Common velvetgrass seedlings develop rapidly, making them competitive at this early stage. After 20 weeks of growth in a greenhouse, common velvetgrass produced the greatest overall root biomass and root:shoot biomass of 6 coastal prairie species grown from seed collected in Marin County, California. Species included in this experiment were bulbous canarygrass (Phalaris aquatica), tall fescue (Schedonorus phoenix), red fescue (Festuca rubra), Oregon bentgrass (Agrostis oregonensis), and purple needlegrass (Nassella pulchra). In the first 5 weeks of growth, common velvetgrass' estimated growth rate exceeded that of any other species. When grown in the presence of others, common velvetgrass seedlings were considered the "least responsive to the presence of a neighbor". The relative yield/neighbor plant was significantly smaller for plants grown with common velvetgrass than with any other species (P<0.05) [140]. In greenhouse and field experiments conducted in British Columbia, common velvetgrass grew most rapidly, was most abundant, and resisted invasion best as a seedling. When transplanted as tillers from 11-year-old and 49-year-old pastures, common velvetgrass growth, abundance, and resistance to invasion were lower [143].
Associated vegetation effects: While the growth of common velvetgrass can decrease in the presence of associated vegetation, decreases may not occur at the seedling stage. After the 1st year of growth in a California coastal prairie field study, common velvetgrass produced the greatest shoot biomass of any species when grown in a monoculture and when grown with an equal proportion of native grasses. Aboveground biomass of common velvetgrass decreased in the 2nd and 3rd years of the experiment (Corbin and D'Antonio, in preparation, cited in [140]). In the Danebo Wetlands of West Eugene, Oregon, the relative performance of common velvetgrass was lower in mixed species field plots than in monoculture field plots. Two years after seeding, average common velvetgrass biomass in a monoculture was 10.2 to 19.6 g/plant and in mixed plots was 0.45 to 1.33 g/plant. Biomass values reported are only for those plots in which common velvetgrass established [36].
Germination date: In both native and nonnative habitats, earlier germination dates are associated with increased seedling establishment, growth, and survival of common velvetgrass. In the United Kingdom, earlier sowing dates related to increased common velvetgrass establishment in the field. Sowing began on 19 July and continued at weekly intervals (Mortimer 1974, as cited in [157]). In field studies conducted in western Oregon, common velvetgrass growth and survival were best at the earliest fall planting date [63,64].
Common velvetgrass survival and growth at different planting dates [63,64] Sowing date Sept. 26 Oct. 7 Oct. 16 Oct. 25 Nov. 8 Seedlings survivingShade effects: Increasing shade reduced seedling growth and survival during field studies in western Oregon. In 0%, 33%, 53%, and 78% shade, 5- to 6-week-old common velvetgrass seedlings had dry weights of 9.2 mg, 3.8 mg, 2.5 mg, and 2 mg, respectively. Shading of 33% or greater significantly reduced seedling growth (P<0.05) [63,64].
Moisture: While moisture may increase seedling survival and establishment, both drought and long periods of inundation can decrease seedling establishment. Added spring moisture increased seedling establishment and survival in an annual coastal prairie in the University of California's South Meadow in Mendocino County. Control, winter-irrigated, or spring-irrigated plots (each 900 cm ²) were seeded in fall. Irrigation treatments added 17 inches (42 cm) of water. Common velvetgrass seedling establishment was 80% on spring-irrigated plots, 55% on winter-irrigated plots, and 30% on control plots. Of the 28 common velvetgrass seedlings that survived most of the subsequent summer drought and growing season, 1 occurred on the control plot, 4 on the winter-irrigated plot, and 23 on the spring-irrigated plot. On spring-irrigated plots, 17 common velvetgrass plants flowered and were described as "large" and "robust" [139]. In field experiments in the Danebo Wetlands common velvetgrass established in plots inundated for 12 to 14 weeks from January to June but failed to establish in plots inundated for 24 to 27 weeks during the same period [36].
Herbivory and disturbance effects: In native habitats, studies indicate that disturbances can increase common velvetgrass seedling establishment and survival, but the effects of simulated and natural herbivory on seedling establishment and survival were mixed. Mechanical soil disturbances may increase establishment more than canopy removal. In a fen meadow and a rush (Juncus spp.) pasture in Devon, southwestern England, common velvetgrass seedling emergence and survival generally increased with disturbances that involved inversion of the top 2.8 inches (7 cm) of soil. In the pasture, clipping dramatically decreased common velvetgrass seedling surival but in the fen, seedling survival was not affected by clipping. Common velvetgrass seedling survival was greatest in the fen with soil disturbance alone and in the pasture with soil disturbance and irrigation and without clipping. Field conditions were "exceptionally dry" in June and July [74].
Emergence and survival of common velvetgrass seedlings on treatment plots with and without irrigation, canopy vegetation, and soil disturbance [74] Treatment Seedling emergence (%) Seedling survival May-October* (%) Irrigation Clipping Soil disturbance Fen Pasture Fen Pasture - - - 18 16 97 75 - - + 12 21 100 96 - + - 12 2 70 0 - + + 21 25 58 35 + - - 17 18 93 98 + - + 20 23 98 100 + + - 11 1 60 0 + + + 23 19 90 0 *Researchers noted that conditions were "exceptionally dry" in June and July.The absence of vegetation cover reduced the probability of successful seedling establishment in a field study in the Treborth Botanic Garden of North Wales. The fate of 275 common velvetgrass seeds was tracked for 8 months on a plowed plot, an herbicide-treated plot, and a relatively undisturbed plot. Just 37% of the seeds developed into seedlings. The probability of survival to adulthood was much lower. Probablity of establishing an adult was 0.0329 on the plowed plot, 0.0333 on the undisturbed plot, and 0.0048 on the herbicide-treated plot when small mammals, birds, and invertebrates were not excluded. Probablity of establishing an adult increased to 0.0518, 0.0592, and 0.0148 on plowed, undisturbed, and herbicide-treated plots, respectively, when small mammals, birds, and invetebrates were excluded [100].
Four years of rabbit exclusion increased common velvetgrass cover, flowering, and seedling emergence but not seedling survival in an acidic, species-poor grassland in Silwood Park, Berkshire, United Kingdom. Common velvetgrass cover, flower production, and seedling emergence from soil samples in rabbit-exclusion plots were more than double that of unfenced plots; however, the proportion of common velvetgrass seedlings surviving from fall 1995 to mid-winter 1997 was 0.18 in exclusion plots and 0.32 in unfenced plots. All differences were significant (P<0.01) [43].
Additional information on common velvetgrass seedling establishment, plant growth, and spread is available in Impacts.
While often a component of newly disturbed, open communities, common velvetgrass may also occur in stable savannas or heavily shaded mid- and late-seral forest types. In a review, Beddows [14] reported that common velvetgrass "readily colonizes bare soil and disturbed ground", but that plant size and abundance often decrease with increasing severity of common velvetgrass defoliation. Grime [58] classified common velvetgrass as a "competitive ruderal" that is often present as a seedling in the colonization of bare ground but is typically most abundant when disturbances are "less immediate or catastrophic". Researchers in British Columbia described common velvetgrass as "scattered to plentiful" in early-seral communities and/or disturbed sites [83]. In the Puget Trough of Washington, common velvetgrass is typical in Douglas-fir–Pacific madrone/pink honeysuckle vegetation if grazed in the past or near a severely disturbed area [25]. In grasslands and savannas of Hawaii Volcanoes National Park, common velvetgrass established soon after and was often abundant on recent pig digs and artificially created disturbances [129]. Disturbance-related succession is discussed below.
Shade relationships: Although some consider common velvetgrass shade intolerant [83] and studies have shown that shade may decrease seedling growth and plant biomass [52,63], common velvetgrass may occur in heavily shaded woodlands with high tree density [90], especially with soil disturbance.
Field studies in western Oregon revealed that increasing shade reduced common velvetgrass seedling growth and survival [63,64]. For details, see Shade effects. In dune grasslands in the Newborough Warren National Nature Reserve of Wales, the density of common velvetgrass was 0.6 plants/200 cm² in plots shaded by surrounding vegetation and 2.6 plants/200 cm² in plots where vegetation was held back. Total common velvetgrass biomass was 0.02 g/200 cm² in shaded plots and 1.1 g/200 cm² in unshaded plots [52]. When the vegetation and environmental data were analyzed for 184 plots in Oregon white oak savannas in Vancouver Island's Cowichan Valley, common velvetgrass was most frequent on partly shaded sites. On a scale from 0 (completely shaded) to 1 (completely unshaded), common velvetgrass' shade preference ranked near 0.3 [91]. On the Hoh River in Washington's Olympic National Park, common velvetgrass cover and frequency were 4.4% and 30%, respectively, in 14-year-old red alder stands; less than 1% and 3% in 24-year-old stands; and common velvetgrass was absent from 65-year-old stands. Shading was heaviest and tree density greatest in 14-year-old stands; canopy openness increased with increasing stand age [90].
Hydrarch succession: Common velvetgrass is typically found in the last and driest stages of hydrarch succession of temporary ponds in the Willamette Valley. Common velvetgrass occurred in the "grassland-composite" stage that appeared only after water levels decreased with root and litter accumulations of submerged and emergent vegetation [89].
Old-field succession: Pastures and abandoned fields are important common velvetgrass habitats. As succession proceeds to shrublands, woodlands, and forests, common velvetgrass may become less frequent. On pastures near Aldergrove, British Columbia, common velvetgrass cover was 9.5% on a 2-year-old pasture, 20.6% on a 21-year-old pasture, 37.9% on a 40-year-old pasture, and 15.1% on a 65-year-old pasture (Aarssen 1983, as cited in [134]). In the northeastern United States, common velvetgrass decreased in frequency with increased time since last disturbance. On an abandoned agricultural field that was last cultivated in 1945 and last grazed in 1951, the frequency of common velvetgrass was 60% in 1954, 48% in 1960, 10% in 1973, and was absent after that. Vegetation of the old field changed from an open perennial meadow in 1954 to a shrub-dominated thicket in 1973 and to a young hardwood forest or woody vine community by 1992 [45].
Forest Succession: Common velvetgrass is possible in heavily shaded, shrub- or hardwood-dominated seral forests but rarely occurs in old-growth forests without disturbance. Many studies from Oregon and Washington indicate that common velvetgrass is typical in the understory of red alder stands and thickets. In these studies, red alder stands with common velvetgrass ranged from 2 to 75 years old ([47,61,68], Henderson 1970, as cited in [49]). While common velvetgrass occurred in the understory of red alder and Scouler willow (Salix scouleriana) thickets on recent river terraces of the Hoh River in Olympic National Park, it did not occur in forest-dominated terraces [47]. In the lower Fraser Valley and southern Vancouver Island, British Columbia, common velvetgrass was frequent in western hemlock/goose neck moss (Tsuga heterophylla/Rhytidiadelphus loreus) forests logged less than 5 years previously and in stages prior to closure of the sapling canopy. Frequency was lower in the closed-canopy sapling phase, and common velvetgrass was lacking in late immature pole stands, mature stands, and old-growth stands [84].
Disturbance-related succession: Common velvetgrass is often more abundant on disturbed than undisturbed sites. As time since disturbance increases, common velvetgrass abundance often decreases.
Logging: An increased occurrence of common velvetgrass is common following forest logging operations. Increases may be greater on more heavily disturbed sites. Soil scarification may be more important to common velvetgrass establishment and growth than canopy removal. Common velvetgrass was more common in the understory of thinned than untreated Douglas-fir forests on Washington's Ft. Lewis Military Reservation. One year after thinning, the indicator value of common velvetgrass was 36 on the thinned site and 0 on the untreated site. Three years after thinning, the indicator value of common velvetgrass was 59 on thinned and 2 on untreated sites. Average cover of native woody species was not much different on thinned (41.9%) and untreated (45.8%) stands 3 years after thinning [142]. Common velvetgrass occurred in Douglas-fir/western sword fern-redwood-sorrel (Polystichum munitum-Oxalis oregana) habitat types in the southern Oregon Coast Range 4 to 25 years after clearcutting. Common velvetgrass cover was greater on severely disturbed than on relatively undisturbed clearcuts. Severely disturbed sites experienced soil disturbances from skid trails and other operations [7]. Common velvetgrass frequency was much greater on unburned clearcut sites than on burned clearcut sites in other Douglas-fir forests in the Oregon Coast Range. Unburned sites were evaluated 3 years after clearcutting, and burned sites were evaluated 2 years after slash burning. More on this study is presented in Fire adaptations and plant response to fire [30].
Grazing: Reviews report that common velvetgrass is susceptible to trampling [158] and that plant size and abundance often decrease with increasing defoliation severity. In an England pasture, common velvetgrass seedling establishment and survival were much lower on clipped sites than on undisturbed sites or sites with mechanically disturbed soils [74]. In several western US studies, common velvetgrass abundance or growth was less on grazed or clipped than on ungrazed or unclipped sites [1,65,66,124,144].
Although susceptible to trampling [158], decreases in common velvetgrass abundance may be short-lived. When common velvetgrass in a North Carolina old-field was trampled up to 500 times by people wearing lug-soled boots, relative cover of common velvetgrass was 32% two weeks after trampling but was 85% a year after trampling [28].
When 42 paired grazed and ungrazed sites were compared in central coastal California prairies, common velvetgrass cover was substantially less on cattle-grazed than ungrazed sites. On sites visited in 2000, common velvetgrass cover averaged 10.8% on grazed and 23.7% on ungrazed sites. For sites visited the next year, common velvetgrass cover averaged 8.4% on grazed and 36.5% on ungrazed sites. Differences were significant on sites visited in 2001 (P<0.01). Precipitation levels were slightly below normal for the study period [65,66]. On the Tomales Point Elk Reserve in Marin County, California, elk grazing reduced abundance of common velvetgrass in open grasslands, but abundance was not reduced when plants grew beneath coyote bush (Baccharis piluaris). Poor accessibility was likely the reason for reduced grazing beneath shrubs [75]. In perennial grasslands of northwestern California, the cover of common velvetgrass was 0.1% in perennial grasslands grazed by cattle for 8 months of the year and 1.7% in grasslands grazed for 4 months of the year. Cover was nearly equal on Oregon oak woodland sites grazed for 8 and 4 months [124]. In a greenhouse study, increased exposure to grazing appeared to improve common velvetgrass' regrowth following clipping. Plants were collected from 2-year-old, 21-year-old, and 40-year-old pastures in the lower Fraser Valley of British Columbia. In general, biomass production was lower for clipped than unclipped plants, but clipped plants produced more tillers than unclipped plants. Total biomass, shoot biomass, and tiller number of clipped plants from the oldest pasture were significantly greater (P<0.05) than those of clipped plants from 2- and 21-year-old pastures. Common velvetgrass from the oldest pasture was exposed to grazing pressure for the longest time. Differences in common velvetgrass regrowth between the oldest and younger pastures suggest that plants developed a tolerance to grazing on the oldest pasture [1].
Other: A variety of disturbances can impact the establishment and spread of common velvetgrass. Generally open sites with favorable moisture provide for the best common velvetgrass establishment and growth; however, establishment and growth vary in their response to shading, disturbance, and fertility. In a British Columbia field experiment, researchers found that common velvetgrass growth was greatest in undisturbed plots with low-nutrient levels. Monoculture and mixed stands were established from seed collected in pastures. Seedlings were grown for 11 months before applying treatments and evaluated after 5 months of nutrient additions and/or clipping treatments. Common velvetgrass cover was greatest in undisturbed monocultures with low-nutrient levels. Clipping to 0.4-inch (1 cm) heights each week was the highest level of disturbance tested and severely depressed common velvetgrass growth. Cover of common velvetgrass was lowest in high-disturbance, low-nutrient, mixed-species treatments [144].
Studies conducted in Derbyshire, United Kingdom, suggest that common velvetgrass establishment and abundance may increase with increased disturbance and fertility. Five years after common velvetgrass was seeded along fertility and disturbance gradients, researchers indicated that common velvetgrass "appeared to respond positively to both increased fertility and disturbance". Disturbances involved turf removal [137]. At the conclusion of this experiment, 3 years after 3 years of treatments, common velvetgrass cover differences were greatest between fertile, disturbed plots (30.3%) and infertile, undisturbed plots (9.0%) (P=0.001). Researchers reported that common velvetgrass went into "relatively steep" decline after treatments were discontinued [23].
Common velvetgrass appeared early after debris flows in the Coast Range of central Oregon and on Mount St Helens in Washington. On the more severe Mount St Helens debris flows, common velvetgrass increased consistently with time since flow [35]. On the less severe debris flow in Oregon, common velvetgrass increased until about the 4th year after the flow [102].
In alpine hairgrass grasslands and koa savannas in Hawaii Volcanoes National Park, common velvetgrass established soon after and was often abundant on recent pig digs and artificially created disturbances. Researchers concluded that pig digging could "greatly enlarge" the abundance of nonnative species in mostly native communities [129]. However, in the montane rainforest zone of Hawaii Volcanoes National Park, common velvetgrass was not associated with feral pig disturbances. Researchers suggested that common velvetgrass establishment and growth may have been so rapid that disturbances were not recognized as recent [4].
In coastal prairie vegetation in Sonoma County, California, common velvetgrass establishment and growth were greater in canopy gaps created in sweet vernalgrass patches than in common velvetgrass patches. Gaps in the canopy were created by killing individual bunchgrasses; standing dead vegetation remained. By the second year after gap creation in sweet vernalgrass, common velvetgrass cover was 100%. Common velvetgrass leaf area was 1,000 times greater in sweet vernalgrass than in common velvetgrass patches [109].
Common velvetgrass clumps expand through tillering or the growth and development of prostrate rosette shoots (Tansley 1949, as cited in [14]). In reviews, vegetative growth of common velvetgrass has been described as producing a "blanket of runners or stolons" on the soil surface [158] and as "aggressive tillering" that allows clumps to "enlarge rapidly" [149]. Claims of rhizome production [40] and sprouting following top-kill [51] were not substantiated by the available literature.
While vegetative growth commonly occurs, recruitment of seedlings is typically the primary method of common velvetgrass reproduction. In field and greenhouse studies conducted in British Columbia, seedlings grew more rapidly and resisted invasion better than tillers collected from 11- and 49-year-old pastures. In the greenhouse, common velvetgrass was least abundant in patches grown from tillers collected in the 49-year-old pasture and most abundant in seeded patches. Common velvetgrass seedling patches were the most difficult to invade by other nonnative pasture grasses, but patches grown from tillers collected in the 11- and 49-year old pastures were invaded easily. In the field, common velvetgrass tiller patches expanded at a rate of 8.28 cm²/week, while seedling clumps expanded at a rate of 16.0 cm²/week [143].
In frequently mowed habitats, the importance of tillering increases. In the North Wales Treborth Botanic Garden, nearly all species, including common velvetgrass, colonized cleared patches through vegetative growth in frequently mowed grassland (1-2 times/2 weeks). Seedlings (of any species) were extremely rare [5]. At 4 weeks old, common velvetgrass tillers can survive apart from the parent plant. Researchers collected common velvetgrass plants from a pasture in Cheshire, United Kingdom, potted them in a greenhouse and evaluated the survival of severed tillers. Four-week old tillers survived, but 1- and 2-week old tillers did not [24].
Vitola (Holcus lanatus ) ye una especie de la familia de les poacees. Nativa d'Europa y naturalizada en sitios de clima templáu d'otros continentes.
Yerba perenne, cespitosa, selemente pelosa. Tarmos erectos, de 20-80 (-100) cm d'altor. Fueyes lliniares, planes de 3-10 mm d'anchor. Flores en panícula espiciforme o piramidal, de variable densidá, d'hasta 15 (-20) cm de llargor; espiguillas lateralmente estruyíes, toes fértiles, ovoidees, frecuentemente tiñíes de púrpura, con 2 o 3 flores; lema con aresta subapical; pálea membranosa. Frutu del mesmu tipu que les ceberes (cariopsis). Floria en primavera y branu. Especie fuertemente alergógena.[1]
Praos de siega y praderíes permanentes con agua disponible la mayor parte del añu. Holcus lanatus nel so hábitat natural ye una fonte d'alimentu pa la caparina Pararge aegeria.
Holcus lanatus describióse por Carlos Linneo y espublizóse en Species Plantarum 2: 1048. 1753.[2]
Holcus tuberosus Salzm. ex Trin.[4][5]
Saboya,cañota, segáu, segáu blancu, galhopita, patablranca.
 Esta páxina forma parte del wikiproyeutu Botánica, un esfuerciu collaborativu col fin d'ameyorar y organizar tolos conteníos rellacionaos con esti tema. Visita la páxina d'alderique del proyeutu pa collaborar y facer entrugues o suxerencies.
Esta páxina forma parte del wikiproyeutu Botánica, un esfuerciu collaborativu col fin d'ameyorar y organizar tolos conteníos rellacionaos con esti tema. Visita la páxina d'alderique del proyeutu pa collaborar y facer entrugues o suxerencies. Vitola (Holcus lanatus ) ye una especie de la familia de les poacees. Nativa d'Europa y naturalizada en sitios de clima templáu d'otros continentes.
Planhigyn blodeuol Monocotaidd a math o wair yw Maswellt penwyn sy'n enw gwrywaidd. Mae'n perthyn i'r teulu Poaceae. Yr enw gwyddonol (Lladin) yw Holcus lanatus a'r enw Saesneg yw Yorkshire fog.[1] Ceir enwau Cymraeg eraill ar y planhigyn hwn gan gynnwys Maswellt, Cawnen Benwen, Maeswellt Sypwraidd, Maswellt Sypwraidd, Maswellt Sypwreiddiog.
Gall dyfu bron mewn unrhyw fan gan gynnwys gwlyptiroedd, coedwigoedd a thwndra. Dofwyd ac addaswyd y planhigyn gan ffermwyr dros y milenia; chwiorydd i'r planhigyn hwn yw: india corn, gwenith, barlys, reis ac ŷd.
Planhigyn blodeuol Monocotaidd a math o wair yw Maswellt penwyn sy'n enw gwrywaidd. Mae'n perthyn i'r teulu Poaceae. Yr enw gwyddonol (Lladin) yw Holcus lanatus a'r enw Saesneg yw Yorkshire fog. Ceir enwau Cymraeg eraill ar y planhigyn hwn gan gynnwys Maswellt, Cawnen Benwen, Maeswellt Sypwraidd, Maswellt Sypwraidd, Maswellt Sypwreiddiog.
Gall dyfu bron mewn unrhyw fan gan gynnwys gwlyptiroedd, coedwigoedd a thwndra. Dofwyd ac addaswyd y planhigyn gan ffermwyr dros y milenia; chwiorydd i'r planhigyn hwn yw: india corn, gwenith, barlys, reis ac ŷd.
Fløjlsgræs (Holcus lanatus) er et 20-60 cm højt græs med tueformet vækst. Det er meget almindeligt i Danmark på fugtige enge og ved vejkanter. Planten tåler og trives endda godt med hyppig slåning eller nedbidning
Stænglerne er opstigende eller helt oprette, dunhårede og rødligt eller violet årede ved grunden. Hver stængel har 2-3 knæ. Bladskederne er runde (”oppustede”) og nøgne eller tyndt behårede. Bladene er linjeformede og tilspidsede, flade og tæt dunhårede. Begge bladsider er grågrønne til hvidligt grønne.
Blomstringen sker i juni-juli, hvor man finder blomsterne siddende i småaks, der er samlet i toppe, som er meget tætte og hvidlige. Småakset har to blomster: en nedre, som er tvekønnet, og en øvre, der er hanlig. I modsætning til krybende hestegræs har stakken ved modenhed en krogformet spids og er indeholdt i småakset. Frugterne er nødder. Størrelsen af Fløjlsgræs kan angives som 0,50 x 0,25 m (50 x 25 cm/år).
Rodnettet består af en tæt klump af trævlerødder. [1].
Fløjlsgræs er udbredt i Nordafrika, Mellemøsten, Kaukasus og Europa. I Danmark er den især almindelig på mager jord.
På våde enge, som bliver slået regelmæssigt, findes arten i Danmark sammen med bl.a. hjertegræs, vibefedt, europæisk engblomme, blåtop, bredbladet kæruld, butblomstret siv, dyndpadderok, engrottehale, engrævehale, engsvingel, engtroldurt, gul fladbælg, gul frøstjerne, gul star, gåsepotentil, kantet kohvede, katteskæg, kærtidsel, kærtrehage, majgøgeurt, rød kløver, skedestar, trævlekrone og vild hør [1].
Fløjlsgræs (Holcus lanatus) er et 20-60 cm højt græs med tueformet vækst. Det er meget almindeligt i Danmark på fugtige enge og ved vejkanter. Planten tåler og trives endda godt med hyppig slåning eller nedbidning
Das Wollige Honiggras (Holcus lanatus) ist eine Pflanzenart, die zur Familie der Süßgräser (Poaceae) gehört.[1] Sie ist in Eurasien und Nordafrika weitverbreitet.
Regionale Trivialnamen sind Bottermeddel, Honigmeddel, Honigschmale, Pein, Sametschmale, Witten Meddel oder Zuckerschmale.
Das Wollige Honiggras ist eine überwinternd grüne[1], ausdauernde krautige Pflanze, die Wuchshöhen zwischen 20 und 100 Zentimeter erreicht. Alle oberirdischen Pflanzenteile sind dicht wollig behaart. Es wächst locker bis dichthorstig mit aufrechten oder von gebogenem Grund aufsteigenden Halmen. Der untere Teil des Stängelgrundes ist auf weißlichem Untergrund rötlich-violett geadert. Die Halme sind dünn und verfügen über zwei bis drei flaumig behaarte Knoten.
Die wechselständig am Halm verteilten Laubblätter sind in Blattscheide und Blattspreite gegliedert. Die Blattscheiden sind auf dem Rücken rundlich, kahl oder wenig behaart, dann mit zurückgeschlagenen Haaren. Die Blatthäutchen (Ligulae) sind häutig, grob gefranst, stumpf und etwa 1 bis 5 Millimeter lang. Die flache Blattspreite ist bei einer Länge von 4 bis 20 Zentimeter und einer Breite von bis zu 10 Millimeter zugespitzt. Die Laubblätter sind sehr dicht kurzhaarig daher graugrün oder weißlich grün aussehend.
Die Blütezeit liegt zwischen Mai und August. Die weißlich bis purpurnfarbenen, nur während der Blütezeit geöffneten Blütenrispen sind schmal-oval oder eiförmig, sehr dicht bis locker und bis 10 Zentimeter lang. Die Rispenäste sind behaart, dicht verzweigt. Die länglichen bis elliptischen, 4 bis 6 Millimeter langen Ährchen fallen geschlossen bei der Reife ab. Die Ährchen sind zweiblütig, die untere Blüte ist zwittrig, die obere gewöhnlich männlich. Die papierartigen Hüllspelzen sind so lang wie das Ährchen und sind an den Kielen und Nerven steif behaart. Die untere ist schmal-lanzettlich und einnervig; die obere eiförmig bis elliptisch, dreinervig und gewöhnlich mit einer etwa 1 Millimeter langen Granne. Die Deckspelzen sind zwischen 2 und 2,5 Millimeter lang und werden von den Hüllspelzen völlig eingeschlossen. Sie sind aufwärts gekielt, undeutlich drei- bis fünfnervig, fest und glänzend. Die untere ist bootsförmig stumpf und unbegrannt; die obere ist auf dem Rücken nahe der Spitze begrannt. Diese Granne wird bis zu 2 Millimeter lang und krümmt sich in trockenem Zustand wie ein Angelhaken, ragt jedoch nicht aus dem Ährchen hervor. Es werden Karyopsen gebildet.
Die Chromosomenzahl beträgt 2n = 14.[2]
Sehr ähnlich ist das insgesamt spärlicher behaarte Weiche Honiggras (Holcus mollis). Dieses Gras verfügt über lange, zähe Rhizome und die Halmknoten sind bärtig behaart. Die Grannen an den oberen Deckspelzen sind nur leicht gebogen und ragen aus den Hüllspelzen heraus. Der Stängelgrund ist auf bräunlichem Untergrund rötlich-bräunlich geadert. Im Gegensatz zum Wolligen Honiggras wächst es bevorzugt an Waldrändern und an lichten Waldstellen.
Im Allgemeinen wird das häufige Gras landwirtschaftlich als Weideunkraut betrachtet. Auf der Weide und im Heu wird es vom Vieh verschmäht, da es stark behaart ist. Auf ärmeren Böden kommt jungen Pflanzen jedoch ein gewisser Ertragswert zu.
Das Wollige Honiggras ist ein teilweise wintergrüner, frostempfindlicher Hemikryptophyt und eine Horstpflanze. Es ist eine Humuswurzler mit VA-Mykorrhiza.[3][4]
Die Blüten sind selbststeril. Blütenökologisch handelt es sich um Windblütigkeit vom „Hängeblütigen-Typ“.[3][4]
Die Diasporen (Ausbreitungseinheit) sind die bei der Reife als Ganzes abfallenden Ährchen; sie breiten sich als Windstreuer, Ballonflieger, Schwimmer, Regenschwemmlinge und Wasserhafter aus. Die bis 2 mm lange Granne der oberen Deckspelze ist im trockenen Zustand wie ein Angelhaken gekrümmt und erlaubt so eine Ausbreitung als Kletthafter. Außerdem erfolgt Zufallsausbreitung durch Weidetiere. Die Karyopsen sind Lichtkeimer. Die Fruchtreife erfolgt von Juli bis November.[3][4]
Das Wollige Honiggras ist in ganz Europa, in West-Asien, Nordafrika und Makaronesien verbreitet. Es ist inzwischen in Nordamerika und anderen Gebieten der Welt mit gemäßigtem Klima ein Neophyt. Es ist auch in Deutschland überall häufig und verbreitet vom Flachland bis in Höhenlagen von etwa 900 Metern. In den Allgäuer Alpen steigt es im Tiroler Teil zwischen Jungholz und Sorgschrofen bis zu 1150 Metern Meereshöhe auf.[5]
Es ist vergleichsweise anspruchslos und wächst auf nahezu allen trockenen bis nassen Böden von schweren Lehmen bis zum Sand. Sein Verbreitungsschwerpunkt liegt in Feuchtwiesen und Weiden auf grundfeuchten, humosen, mäßig nährstoffreichen, leicht sauren Lehm- und Tonböden. In tieferen Lagen kommt es vorwiegend in Sumpfdotterblumenwiesen (Calthion) vor. In höheren Lagen ist es auch in Glatthafer-Gesellschaften (Molinio-Arrhenatheretea) zu finden. Zuweilen wächst es auch in Zwergstrauchheiden und Borstgrasrasen, Trocken- und Halbtrockenrasen sowie in Laub- und Nadelwäldern auf sauren, nährstoffarmen Böden. Die Art ist Klassencharakterart der Europäischen Wirtschaftswiesen und Wirtschaftsweiden, Molinio-Arrhenatheretea.[2]
Das Wollige Honiggras (Holcus lanatus) ist eine Pflanzenart, die zur Familie der Süßgräser (Poaceae) gehört. Sie ist in Eurasien und Nordafrika weitverbreitet.
Regionale Trivialnamen sind Bottermeddel, Honigmeddel, Honigschmale, Pein, Sametschmale, Witten Meddel oder Zuckerschmale.
Holcus lanatus is a perennial grass. The specific epithet lanatus is Latin for 'ooie' that descrives the plant's hairy textur. Common names include hose-gress, midge-gress, an pundy gress.
Ruad toost(er) (Holcus lanatus) as en plaantenslach uun det famile swetgäärs (Poaceae).
Ullhært legugras (frøðiheiti Holcus lanatus) er 20-60 cm høg. Sera vanligt í nýlendi og opinektrum; verður nevnt frægras. Stráini saman í túgvum. Øll plantan mjúkhærd, grálig. Tyssið samanliggjandi, áður enn blómingin kemur, men útsperrað seinni. Tað er vanligast reydligt á liti, ljósari og dimmari, stundum næstan hvítt. Niðara aksøgnin er kubbut, men ber ein evurslítlan brodd. Kallblómuøgnin ber eisini ein lítlan brodd. Øll plantan er klødd við mjúkum hárum.
Blómuskipanin eitur eitt tyssi, tí at blómuleggirnir greina seg í smærri greinar, sum bera hvør sína blómu. Tyssið liggur saman, men tá ið plantan blómar, breiðir tyssið seg út. Tyssið er reydligt á liti. Tyssið 3-20 cm, dimmreytt, stundum nakað grønligt ella ljóst, 2 ella 3 blómur í smáaksinum. Niðara blóman tvíkynjað við ongum broddi, tann ovara einkynjað kallblóma við broddi, sum ikki røkkur út úr smáaksinum. Blómar í juni-august. Veksur á nýlendi serliga, sera vanlig innangarðs, men veksur eisini uttangarðs, har ið taðað er.
Ullhært legugras (frøðiheiti Holcus lanatus) er 20-60 cm høg. Sera vanligt í nýlendi og opinektrum; verður nevnt frægras. Stráini saman í túgvum. Øll plantan mjúkhærd, grálig. Tyssið samanliggjandi, áður enn blómingin kemur, men útsperrað seinni. Tað er vanligast reydligt á liti, ljósari og dimmari, stundum næstan hvítt. Niðara aksøgnin er kubbut, men ber ein evurslítlan brodd. Kallblómuøgnin ber eisini ein lítlan brodd. Øll plantan er klødd við mjúkum hárum.
Blómuskipanin eitur eitt tyssi, tí at blómuleggirnir greina seg í smærri greinar, sum bera hvør sína blómu. Tyssið liggur saman, men tá ið plantan blómar, breiðir tyssið seg út. Tyssið er reydligt á liti. Tyssið 3-20 cm, dimmreytt, stundum nakað grønligt ella ljóst, 2 ella 3 blómur í smáaksinum. Niðara blóman tvíkynjað við ongum broddi, tann ovara einkynjað kallblóma við broddi, sum ikki røkkur út úr smáaksinum. Blómar í juni-august. Veksur á nýlendi serliga, sera vanlig innangarðs, men veksur eisini uttangarðs, har ið taðað er.
Holcus lanatus is a perennial flowering plant in the grass family Poaceae. The specific epithet lanatus is Latin for 'woolly' which describes the plant's hairy texture. Common names include Yorkshire fog, tufted grass, and meadow soft grass. In North America, where it is an invasive species,[1] names include velvet grass and common velvet grass.[2][3]
In parts of northern Europe the grass is a common native species and a hardy pasture grass.

Holcus lanatus has velvety grey-green leaves. The stems are round. The bases of the stems are white with pink stripes or veins; this character has been called the "stripy pyjamas".[4] The inflorescence is robust and often tinged purple. It produces a large amount of seed and is a rapid coloniser of disturbed ground. It prefers wetter ground; it is often seen around drainage ditches. The ligule is 1–4 millimetres (0.039–0.157 in) long, blunt, and hairy.
This species can be distinguished from H. mollis by the beardless nodes on its culm, the absence of rhizomes, and the awn becoming hooked when dry and not projecting beyond the tips of the glumes.[2] It has been known to hybridize with H. mollis, producing a male sterile hybrid with 2n = 21 chromosomes.[2] Hybrids tend to resemble H. lanatus in their morphology.[3]
It spreads vegetatively by developing new shoots and roots at its nodes. Plants form a blanket of runners on the soil surface. Semi-prostrate rosettes of shoots called 'mops' may form at the end of the runners. These mops root readily in contact with moist soil.[3]
In a European survey of weed contamination in cereal seed in 1970, Holcus lanatus seed was found in 1% of samples. H. lanatus is an indicator of poor soil, low grazing levels, and poor drainage. It is tolerant of a range of soil pH, but grows best between 5.0 and 7.5. It exhibits climatic tolerance over a wide altitude range, but severe frosts can kill it. It does not survive trampling and puddling. It can be controlled in some European locations by increasing available potassium and phosphorus, increasing stock, and improving drainage. These remedies are not as effective in North America.[3]
Holcus lanatus is a significant pest weed in Australia, as it is a winter-growing C3 grass and survives droughts and hot summers as seed. It is distasteful to stock unless it is young and little other plant material is available. The flowers are wind-pollinated and usually out-crossing. The first seeds become viable 5 to 9 days after flowering and all are viable after 20 days. Seeds are shed from in summer and early autumn. One panicle has 100 to 380 seeds, with 177,000 to 240,000 seeds per plant, depending on time of emergence.[3]
In North America, Holcus lanatus is an invasive species in native grasslands and other ecosystems. In Yosemite National Park it is one of nine priority noxious weeds to control for habitat restoration and regenerating native plant balances.[5] It forms dense stands that can exclude other plants.
It is also established in Chile and Australia.
Holcus lanatus in its natural habitat is a food source for butterflies such as the speckled wood, the wall, and especially the small skipper. It is rarely utilized by the Essex skipper. In its native range it may occur in plant associations such as the Juncus subnodulosus–Cirsium palustre fen-meadow habitat.
Holcus lanatus is a perennial flowering plant in the grass family Poaceae. The specific epithet lanatus is Latin for 'woolly' which describes the plant's hairy texture. Common names include Yorkshire fog, tufted grass, and meadow soft grass. In North America, where it is an invasive species, names include velvet grass and common velvet grass.
In parts of northern Europe the grass is a common native species and a hardy pasture grass.
Holcus lanatus, esperante lana holko, estas greso de la subfamilio de Pooideae. Ĝi estas staŭda.
Haroj estas mallongaj kaj abundaj. Spiketoj havas du floroj, kaŝataj per la glumoj, kun mallonga krampoforma aristo. Dum la matureco, la kompleta spiketo falas.
La planto formas grava rozeton kun multaj taloj kaj kun larĝaj folioj.
La plantido estas karakteriza:[1]
Ĝia koloro estas blua. Nervoj de la ingoj estas roz-rugaj. Molaj haroj estas ĉie.
Claviceps purpurea estas parazito de Holcus lanatus.
Holcus lanatus, esperante lana holko, estas greso de la subfamilio de Pooideae. Ĝi estas staŭda.
Holcus lanatus es una especie de la familia de las poáceas. Nativa de Europa y naturalizada en sitios de clima templado de otros continentes.
Hierba perenne, cespitosa, suavemente pelosa. Tallos erectos, de 20-80 (-100) cm de altura. Hojas lineares, planas de 3-10 mm de anchura. Flores en panícula espiciforme o piramidal, de variable densidad, de hasta 15 (-20) cm de longitud; espiguillas lateralmente comprimidas, todas fértiles, ovoideas, frecuentemente teñidas de púrpura, con 2 o 3 flores; lema con arista subapical; pálea membranosa. Fruto del mismo tipo que los cereales (cariopsis). Florece en primavera y verano. Especie fuertemente alergógena.[1]
Prados de siega y praderas permanentes con agua disponible la mayor parte del año. Holcus lanatus en su hábitat natural es una fuente de alimento para Pararge aegeria.
Holcus lanatus fue descrita por Carlos Linneo y publicado en Species Plantarum 2: 1048. 1753.[2]
saboya, cañota, heno, heno blanco, heno blanco de San Ildefonso, henu, hierba macerguera, hierba triga, holco, holco lanudo, pajón, triguera,[6] pasto dulce, pasto miel, pasto chileno, falsa poa.
Holcus lanatus es una especie de la familia de las poáceas. Nativa de Europa y naturalizada en sitios de clima templado de otros continentes.
Vill-mesihein (Holcus lanatus) on kõrreliste sugukonda arvatud taimeliik.
Taim kasvab Eestis saartel ja rannikualal. Teda leidub seal paiguti.[1]
Vill-mesihein (Holcus lanatus) on kõrreliste sugukonda arvatud taimeliik.
Taim kasvab Eestis saartel ja rannikualal. Teda leidub seal paiguti.
Karvamesiheinä (Holcus lanatus) on mesiheinien sukuun kuuluva heinälaji.
Se on monivuotinen, pehmeäkarvainen ja kasvaa mätästävänä. Lehtilavat ovat kolmesta kymmeneen millimetriä leveät ja niiden tupet tiheän valkokarvaisia. Tiheänmallinen röyhy on sinertävänpunaisenkirjava. Kaksikukkaisten tähkylöiden hedekukan ulkohelpeessä on vihne, joka on hyvin lyhyt ja koukkukärkinen verrattuna toiseen suvun lajiin pehmytmesiheinään (Holcus mollis).
Karvamesiheinän alkuperäinen levinneisyysalue on Eurooppa. Laji on Suomessa myöhään saapunut tulokas, jota on löydetty Etelä- ja Keski-Suomesta sekä Lapista.
Karvamesiheinä (Holcus lanatus) on mesiheinien sukuun kuuluva heinälaji.
Se on monivuotinen, pehmeäkarvainen ja kasvaa mätästävänä. Lehtilavat ovat kolmesta kymmeneen millimetriä leveät ja niiden tupet tiheän valkokarvaisia. Tiheänmallinen röyhy on sinertävänpunaisenkirjava. Kaksikukkaisten tähkylöiden hedekukan ulkohelpeessä on vihne, joka on hyvin lyhyt ja koukkukärkinen verrattuna toiseen suvun lajiin pehmytmesiheinään (Holcus mollis).
Karvamesiheinän alkuperäinen levinneisyysalue on Eurooppa. Laji on Suomessa myöhään saapunut tulokas, jota on löydetty Etelä- ja Keski-Suomesta sekä Lapista.
Holcus lanatus
La Houlque laineuse (Holcus lanatus) est une plante herbacée vivace de la famille des Poacées, sous-famille des Pooideae, assez commune en Europe, fréquente dans les prairies naturelles acides, parfois cultivée comme couvert à gibier ou couvre-sol.
Le latin holcus et l'ancien français houlque ou houque désignaient des graminées à épi en panicule[2], du grec holcos (όλκός : qui attire[3]) : tirer ou retirer (une écharde)[4]. L'adjectif laineuse fait référence à sa villosité et à la sensation éprouvée au toucher de l'épi.
C'est une plante de taille moyenne (jusqu'à 80 cm de haut), très velue.
L'épi, d'abord vert pointu et compact, s'étale et passe ensuite au rose violacé.
Organes reproducteurs
Graine
La Houlque laineuse est l'une des plantes dont certaines souches se montrent particulièrement résistantes à l'arsenic[5] (et plus précisément aux arséniates, formes les plus fréquentes de l'arsenic dans les sols, autrefois utilisée dans certains pesticides (herbicides, fongicides, insecticides comme sur les golfs où la houlque est jugée par ailleurs indésirable). Les pollutions à l'arsenic sont plus souvent le fait de résidus de l'industrie ou de munitions de guerre, elles sont parfois naturelles comme dans le cas des eaux de puits du Bangla-Desh[6].
Habituellement, les arséniates (sels de l'acide arsénique H3AsO4, qui est un analogue de l'acide phosphorique H3PO4) sont facilement absorbés par les racines des plantes, et ce sont des poisons violents qui bloquent notamment la synthèse de l'ATP.
Ainsi confondus avec les phosphates par les plantes, ils peuvent contaminer le réseau trophique. Mais quelques espèces ou souches de plantes y ont développé une certaine résistance[7], dont notamment la Houlque laineuse[8],[9],[10],[11],[12]. D'autres herbacées (Deschampsia cespitosa (L.) Beauv[13] et Agrostis capillaris[13]) y présentent aussi une bonne résistance, de même que quelques espèces de fougères métallophytes.
Prairies européennes circumboréales, tempérées et méditerranéennes, bordures de forêts, étangs, chemins, talus (clôture) Elle est considérée comme caractéristique des prairies atlantiques humides à tendance acide (Juncus subnodulosus–Cirsium palustre fen-meadow (en) des auteurs anglo-saxons : marais à Juncus subnodulosus et cirse des marais). On peut cependant la rencontrer en sol acide et sec; elle peut être indicatrice de pelouses métallicoles, on la rencontre des bords de mer jusqu'à la moyenne montagne[14]'[4].
Elle est considérée comme envahissante (invasive) dans certains pays, par exemple en Amérique du Nord ou à La Réunion[15].
Elle n'est pas cultivée par les éleveurs en France, sa valeur alimentaire étant jugée médiocre à moyenne, elle est cependant bien consommée jeune et sa productivité est bonne[16]. Elle est très commune dans les prairies permanentes pâturées peu intensives et les prairies de fauche (elle supporte mal le piétinement) où elle peut même devenir envahissante[16].
Dans le cas de rénovation de prairies elle peut être remplacée par la fléole des prés mieux appréciée du bétail et qui pousse dans les mêmes conditions de sol et de climat.
Holcus lanatus
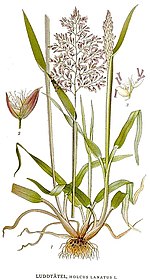
La Houlque laineuse (Holcus lanatus) est une plante herbacée vivace de la famille des Poacées, sous-famille des Pooideae, assez commune en Europe, fréquente dans les prairies naturelles acides, parfois cultivée comme couvert à gibier ou couvre-sol.
Łučna mědlina (Holcus lanatus) je rostlina ze swójby słódkich trawow (Poaceae).
Łučna mědlina je trajne zelo, kotrež docpěwa wysokosć wot 30 hač do 100 cm. Rostlina je šěrozelena a z mjechkimi kosmami přikryta.
Stwjelca su často deleka zhibnjene.
Łopjena docpěwaja šěrokosć wot 3 hač do 8 mm. Jich kožički su trodlate a docpěwaja dołhosć wot 2 mm.
Kćěje wot junija hač do julija (awgusta). Kćenja steja w často čerwjenojće přeběžanych pakićach z dołhosću mjez 6 a 12 cm, kotrež jenož za čas kćěwa su čumpaće rozšěrjene, hewak hromadu ćehnjene. Kłóski su tróšku splacnjene a docpěwaja dołhosć wot 4 hač do 5 mm. Přikrywne pluwizny su běłojte, horjeka čerwjenojte a na šipje a na kromje mikawčkate. Krywne pluwizny su běło błyšćace, při čimž te muskich kćenjow krótki kocht njesu.
Rosće na włóžnych łukach a pastwach, w lěsnych zahonach a w swětłych lěsach.
Rostlina je w Europje rozšěrjena.
Łučna mědlina (Holcus lanatus) je rostlina ze swójby słódkich trawow (Poaceae).
Villainā meduszāle (latīņu: Holcus lanatus) ir daudzgadīgs 30—100 cm augsts lakstaugs. Ne visai bieži sastopams pļavās un sausos uzkalniņos. Veido vairāk vai mazāk blīvus cerus ar nedaudziem stumbriem. Stumbri taisni, pie mezgliem un zem tiem ar mīkstiem, uz leju atliektiem matiņiem. Lapas pamīšus, vienkāršas, veselas, šauri lineāras, 0,2—1 cm platas, nosmailotas, ar izteiktu vidusdzīslu, mīkstas, spilvainas. Lapu makstis vairāk vai mazāk garas, ar mīkstiem, uz leju atliektiem matiņiem, mēlīte līdz 0,2 cm gara. Zied no jūnija līdz augustam. Ziedkopa — blīva, 8—10 cm gara skara ar daudzām vārpiņām. Vārpiņas nelielas, olveidīgi iegarenas, no sāniem saspiestas, ar 2—3 ziediem. Vārpiņas plēksnes tik garas kā vārpiņa, strupi nosmailotas, spilvainas, ar īsu, smailu galotnīti, vārpiņas ārējai plēksnei 1 dzīsla, iekšējai — 3 dzīslas. Vārpiņā apakšējais zieds divdzimumu, zieda ārējā plēksne bez akota, šķautne neizteikta, iekšējai plēksnei šķautne ar dzelonīšiem; augšējais zieds vīrišķais, tā zieda ārējā plēksne ar īsu, āķveida akotu, kas nepārsniedz vai tikko pārsniedz vārpiņu, iekšējās plēksnes bieži vien nav. Auglis — 0,1—0,2 cm garš grauds. Lopbarības ziņā mazvērtīgs augs, jo pūkainuma dēļ mājdzīvnieki to neēd siena veidā. Satur glikozīdus, no kuriem var rasties zilskābe.
Villainā meduszāle (latīņu: Holcus lanatus) ir daudzgadīgs 30—100 cm augsts lakstaugs. Ne visai bieži sastopams pļavās un sausos uzkalniņos. Veido vairāk vai mazāk blīvus cerus ar nedaudziem stumbriem. Stumbri taisni, pie mezgliem un zem tiem ar mīkstiem, uz leju atliektiem matiņiem. Lapas pamīšus, vienkāršas, veselas, šauri lineāras, 0,2—1 cm platas, nosmailotas, ar izteiktu vidusdzīslu, mīkstas, spilvainas. Lapu makstis vairāk vai mazāk garas, ar mīkstiem, uz leju atliektiem matiņiem, mēlīte līdz 0,2 cm gara. Zied no jūnija līdz augustam. Ziedkopa — blīva, 8—10 cm gara skara ar daudzām vārpiņām. Vārpiņas nelielas, olveidīgi iegarenas, no sāniem saspiestas, ar 2—3 ziediem. Vārpiņas plēksnes tik garas kā vārpiņa, strupi nosmailotas, spilvainas, ar īsu, smailu galotnīti, vārpiņas ārējai plēksnei 1 dzīsla, iekšējai — 3 dzīslas. Vārpiņā apakšējais zieds divdzimumu, zieda ārējā plēksne bez akota, šķautne neizteikta, iekšējai plēksnei šķautne ar dzelonīšiem; augšējais zieds vīrišķais, tā zieda ārējā plēksne ar īsu, āķveida akotu, kas nepārsniedz vai tikko pārsniedz vārpiņu, iekšējās plēksnes bieži vien nav. Auglis — 0,1—0,2 cm garš grauds. Lopbarības ziņā mazvērtīgs augs, jo pūkainuma dēļ mājdzīvnieki to neēd siena veidā. Satur glikozīdus, no kuriem var rasties zilskābe.
De gestreepte witbol (Holcus lanatus), ook wel wollig zorggras genoemd, is een wilde, vaste plant uit de grassenfamilie (Poaceae), die een dichte zode kan vormen.
De plant wordt 30-90 cm hoog en zowel het blad als de stengel zijn zacht behaard. De stengels kunnen rechtopgaand of geknikt opstijgend zijn. De plant vormt geen wortelstokken dit in tegenstelling tot gladde witbol (Holcus mollis). Gestreepte witbol bloeit van mei tot september in dichte pluimen.
Het blad is grijsgroen en is voorzien van zachte haartjes. Langs de bladrand zitten lange haren. Elk blad heeft een lengte van maximaal 20 cm en is fijngepunt. Het tongetje (ligula) is tot 4 mm lang en heeft een vlakke top.
De aartjes zijn tot zes mm lang en bestaan uit twee bloemen. De tweede bloem is mannelijk en heeft aan het lemma (kafje) een haakvormig, naar achteren gekromde kafnaald. Het kafnaaldje steekt niet buiten de kelkkafjes uit. De kleur is wit, groen, roze of purper. De samengestelde pluim is aanvankelijk lancetvormig, maar wordt later meer piramidaal. De vrucht is een graanvrucht.
De gestreepte witbol komt voor langs wegen, in grasland, in vochtige duinvalleien, in bos, op onbebouwd terrein en dijken.
In Noord-Amerika is de gestreepte witbol een invasieve soort die zich vestigt in inheemse graslanden en andere ecosystemen. Ze wordt beschouwd als een van de belangrijkste te verdelgen soorten in Yosemite National Park.
De gestreepte witbol (Holcus lanatus), ook wel wollig zorggras genoemd, is een wilde, vaste plant uit de grassenfamilie (Poaceae), die een dichte zode kan vormen.
De plant wordt 30-90 cm hoog en zowel het blad als de stengel zijn zacht behaard. De stengels kunnen rechtopgaand of geknikt opstijgend zijn. De plant vormt geen wortelstokken dit in tegenstelling tot gladde witbol (Holcus mollis). Gestreepte witbol bloeit van mei tot september in dichte pluimen.
Het blad is grijsgroen en is voorzien van zachte haartjes. Langs de bladrand zitten lange haren. Elk blad heeft een lengte van maximaal 20 cm en is fijngepunt. Het tongetje (ligula) is tot 4 mm lang en heeft een vlakke top.
De aartjes zijn tot zes mm lang en bestaan uit twee bloemen. De tweede bloem is mannelijk en heeft aan het lemma (kafje) een haakvormig, naar achteren gekromde kafnaald. Het kafnaaldje steekt niet buiten de kelkkafjes uit. De kleur is wit, groen, roze of purper. De samengestelde pluim is aanvankelijk lancetvormig, maar wordt later meer piramidaal. De vrucht is een graanvrucht.
De gestreepte witbol komt voor langs wegen, in grasland, in vochtige duinvalleien, in bos, op onbebouwd terrein en dijken.
Kłosówka wełnista (Holcus lanatus L.) – gatunek byliny należący do rodziny wiechlinowatych. W Polsce roślina dość pospolita na całym obszarze od niżu po niższe położenia górskie. Gatunek wiatro-obcopylny. Autotrof, hemikryptofit.
Tworzy mieszańce z kłosówką miękką Holcus mollis.
Jest rośliną żywicielską larw motyla karłątka leśnego[3].
Kłosówka wełnista (Holcus lanatus L.) – gatunek byliny należący do rodziny wiechlinowatych. W Polsce roślina dość pospolita na całym obszarze od niżu po niższe położenia górskie. Gatunek wiatro-obcopylny. Autotrof, hemikryptofit.
Holcus lanatus é uma espécie de planta com flor pertencente à família Poaceae.
A autoridade científica da espécie é L., tendo sido publicada em Species Plantarum 2: 1048. 1753.[1]
Os seus nomes comuns são erva-lanar, erva-branca, erva-maior, erva-mansa, erva-molar, erva-mole, erva-serôdia ou nevoeiro-de-yorkshire.[2]
Trata-se de uma espécie presente no território português, nomeadamente os seguintes táxones infraespecíficos:[3]
Holcus lanatus é uma espécie de planta com flor pertencente à família Poaceae.
A autoridade científica da espécie é L., tendo sido publicada em Species Plantarum 2: 1048. 1753.
Os seus nomes comuns são erva-lanar, erva-branca, erva-maior, erva-mansa, erva-molar, erva-mole, erva-serôdia ou nevoeiro-de-yorkshire.
Luddtåtel (Holcus lanatus) är en växtart i familjen gräs.
Luddtåtel (Holcus lanatus) är en växtart i familjen gräs.
Багаторічник. Стебла густо опушені, прямостоячі або колінчасто висхідні, 20–80(-120) см завдовжки. Кореневища відсутні. Листя і піхви від м'яко рідко до щільно опушених. Піхви 1–2,5(-4) мм довжиною. Листові пластинки довжиною 4–20 см, шириною 3–10 мм; середньо зелені або сіро-зелені. Суцвіття — волоть (3-)7–14(-17) см завдовжки, 1–8 см в ширину. Колоски від тупих до підгострих на вершині, еліптичні, стислі з боків; 4–6 мм довжиною.
Північна Африка: Алжир, Марокко, Туніс; Азія: Ліван, Туреччина, Азербайджан, Грузія, Росія; Європа: Білорусь, Естонія, Латвія, Литва, Україна, Австрія, Бельгія, Чехія, Німеччина, Угорщина, Нідерланди, Польща, Словаччина, Швейцарія, Данія, Ірландія, Норвегія, Швеція, Велика Британія, Албанія, Боснія і Герцеговина, Болгарія, Хорватія, Греція, Італія, Румунія, Сербія, Словенія, Франція, Португалія, Іспанія. Натуралізований та культивується в інших частинах світу.
В Україні зростає на луках, в розріджених лісах (переважно суборах), на лісових галявинах, серед кущів — У Поліссі (крім східної частини), західних лісових районах, Карпатах і Південному Криму часто; в Лісостепу рідко; в Степу 1 місце в Комишуваському районі Запорізької області.[1]
Holcus lanatus là một loài thực vật có hoa trong họ Hòa thảo. Loài này được L. mô tả khoa học đầu tiên năm 1753.[1]
Holcus lanatus là một loài thực vật có hoa trong họ Hòa thảo. Loài này được L. mô tả khoa học đầu tiên năm 1753.
Holcus lanatus L. (1753)
СинонимыБуха́рник шерсти́стый (лат. Hólcus lanátus) — типовой вид рода Бухарник (Holcus) семейства Злаки (Poaceae).
Видовой эпитет лат. lanatus в переводе обозначает «шерстистый», что указывает на опушённость растения.
Изначально вид встречался в Европе, умеренных областях Азии и Северной Африке, но был искусственно завезён в Северную Америку и другие регионы с умеренным климатом. В некоторых частях Северной Европы он является обыкновенной пастбищной травой, а в Северной Америке он стал инвазивным видом.
Многолетнее травянистое растение с бархатистыми серо-зелёными листьями. Поросль расходится по кругу. У основания эти отпрыски бело-розовые, с полосками или жилками — эта особенность используется при идентификации. Лигула (язычок) 1—4 мм длиной, с притуплённым концом, опушённая.
Соцветие — крепкий колос или метёлка, часто слегка пурпурная. Ости цветковых чешуй крючковидные и не выступают за концы колосковых чешуй.
Растение производит огромное количество семян и быстро заселяет окружающую землю.
Бухарник шерстистый отличается от бухарника мягкого (лат. Holcus mollis) по неопушённым узлам соломины, а также отсутствием корневища.
Бухарник шерстистый предпочитает влажную почву и часто встречается по берегам сточных канав.
Размножается вегетативно развитием поросли, а также делением корней в узлах. Растения формируют густую дернину из стелющихся побегов. На конце отпрысков иногда формируются полуподнимающиеся розетки. Они особо хорошо развиваются на увлажнённой почве.
Мужской стерильный гибрид бухарника шерстистого с бухарником мягким имеет 2n = 21 хромосому. По морфологическим чертам гибриды похожи на бухарник шерстистый.
В Европе при обследовании семян зерновых на загрязнённость бухарник шерстистый был найден в 1 % проб. Этот вид является индикатором бедной почвы с небольшим запасом питательных веществ или слабым дренажированием. Он нечувствителен к колебаниям pH почвы, однако лучше всего растёт при pH 5,0—7,5. Демонстрирует климатическую чувствительность на большом диапазоне высот. При определённых условиях для него оказываются губительны сильные морозы. Также он не выносит вытаптывания из-за уплотнения почвы. В некоторых европейских странах удаётся подавить его рост за счёт увеличения в почве доступных калия и фосфора, а также увеличивая питательность почвы и её дренаж, однако этот метод в Северной Америке неэффективен[источник не указан 2407 дней].
В Австралии бухарник шерстистый является одним из главных сорняков. Зимой он растёт, а летнюю засуху переносит на стадии семени. Перекрёстное опыление цветков осуществляется ветром. Через 5-9 дней после цветения семена приобретают всхожесть, а через 20 дней они становятся полностью способными к прорастанию в молодое растение. Семена распространяются с июня до начала осени. Число семян от одного растения колеблется от 177 тыс. до 240 тыс. в зависимости от времени появления.
Бухарник шерстистый является инвазивным видом в естественных пастбищах, а также разрушает другие экосистемы. В Национальном парке Йосемити он является одним из девяти наиболее важных сорняков. На месте своего обитания он образует плотные скопления, не давая расти другим растениям, в том числе может уменьшить численность или устранить даже местные злаки.
В естественных местах обитания бухарник шерститсый является источником пищи для разных бабочек, таких как краеглазка эгерия (лат. Pararge aegeria), буроглазка Мегера (лат. Lasiommata megera) и, особенно, Thymelicus sylvestris, редко — Thymelicus lineola. Может произрастать в различных растительных сообществах, например, болотистых лугах.
Буха́рник шерсти́стый (лат. Hólcus lanátus) — типовой вид рода Бухарник (Holcus) семейства Злаки (Poaceae).
绒毛草(学名:Holcus lanatus)为禾本科绒毛草属下的一个种。
|access-date=中的日期值 (帮助)
 分類 界 : 植物界 Plantae 門 : 被子植物門 Magnoliophyta 綱 : 単子葉植物綱 Liliopsida 目 : イネ目 Poales 科 : イネ科 Poaceae 属 : シラゲガヤ属 Holcus 種 : シラゲガヤ H. lanatus 学名 Holcus lanatus
分類 界 : 植物界 Plantae 門 : 被子植物門 Magnoliophyta 綱 : 単子葉植物綱 Liliopsida 目 : イネ目 Poales 科 : イネ科 Poaceae 属 : シラゲガヤ属 Holcus 種 : シラゲガヤ H. lanatus 学名 Holcus lanatus シラゲガヤ(白毛茅、学名:Holcus lanatus)は、単子葉植物イネ科シラゲガヤ属の多年草の一種。ヨーロッパ原産で、世界中に帰化植物として広がっている。
ヨーロッパ原産だが、アフリカ、アジア、オセアニア、北アメリカ、南アメリカとほぼ全世界に分布している[1]。
草丈20-100cm。全体的に白い軟毛が密生しており、和名の由来にもなっている。近縁種として、やや毛の少ないニセシラゲガヤがいる。走出枝を出さない。
牧草地や芝生、路傍、林縁、荒地、畑地などの日当たりの良い環境に生育する。花期は6-8月。