pt-BR
nomes no trilho de navegação


Candida albicans és un fong diploide (una mena de llevat) i un agent causal d'una infecció oportunista oral i genital en humans.[3][4] Les infeccions fúngiques sistèmiques (fungèmies) han emergit com una important causa de malaltia morbiditat i de mortalitat a pacients amb Immunodeficiència (per exemple la, SIDA, càncer amb quimioteràpia, trasplantament de medul·la òssia). Els biofilms de C. albicans ràpidament es formen a la superfície dels estris mèdics implantables. A més les infeccions relacionades amb els hospitals de pacients que abans no es consideraven com de risc (per exemple, pacients en unitats de cures intensives) han passat a ser una causa de preocupació sanitària major.
C. albicans actua com a comensal i es troba en la flora intestinal i entre els molts organismes que viuen en la boca dels humans i el seu tracte gastrointestinal. Sota circumstàncies normals, C. albicans viu en el 80% de la població humana sense fer danys, malgrat que un sobrecreixement resulta en la candidosi. La candidiasi sovint s'observa en individus amb immunodeficiència com el positius en HIV. La candidiasi també pot ocórrer en la sang i el tracte genital. La candidiasi, és una condició comuna que en persones sense immunodeficiència es guareix fàcilment. Per a infectar el teixit de l'hoste la forma usual de "C. albicans" és unicel·lular reacciona a les pistes mediambientals i es converteix en una forma multicel·lular filamentosa i invasora.[3]
Una de les característiques més interessants del genoma de C. albicans és que hi ha arranjaments dels cromosomes de manera numèrica i estructural per tal de generar diversitat genètica que porten a canvis en el fenotip. Quan s'hagi estudiat completament el genoma de C. albicans es podran comprendre millor aquests canvis.
Ja s'ha seqüenciat el genoma de C. albicans per la soca SC5314.[5][6] També per la soca WO1[7]
En un procés que superficialment sembla un dimorfisme sexual, C. albicans mostra un procés de canvi fenotípic (phenotypic switching), en el qual diferents morfologies cel·lulars es generen espontàniament a través de dues fases.
L'heterozigosi del genoma de Candida és superior al que es troba en altres genomes i incrementa el nombre de proteïnes diferents codificades pel genoma.[8]
Candida albicans és un fong diploide (una mena de llevat) i un agent causal d'una infecció oportunista oral i genital en humans. Les infeccions fúngiques sistèmiques (fungèmies) han emergit com una important causa de malaltia morbiditat i de mortalitat a pacients amb Immunodeficiència (per exemple la, SIDA, càncer amb quimioteràpia, trasplantament de medul·la òssia). Els biofilms de C. albicans ràpidament es formen a la superfície dels estris mèdics implantables. A més les infeccions relacionades amb els hospitals de pacients que abans no es consideraven com de risc (per exemple, pacients en unitats de cures intensives) han passat a ser una causa de preocupació sanitària major.
C. albicans actua com a comensal i es troba en la flora intestinal i entre els molts organismes que viuen en la boca dels humans i el seu tracte gastrointestinal. Sota circumstàncies normals, C. albicans viu en el 80% de la població humana sense fer danys, malgrat que un sobrecreixement resulta en la candidosi. La candidiasi sovint s'observa en individus amb immunodeficiència com el positius en HIV. La candidiasi també pot ocórrer en la sang i el tracte genital. La candidiasi, és una condició comuna que en persones sense immunodeficiència es guareix fàcilment. Per a infectar el teixit de l'hoste la forma usual de "C. albicans" és unicel·lular reacciona a les pistes mediambientals i es converteix en una forma multicel·lular filamentosa i invasora.
Candida albicans je druh kvasinky, jenž někdy u lidí způsobuje ústní a genitální houbové infekce. Tato mykóza je zejména častou příčinou nemocí a úmrtí u osob se sníženou imunitou (vlivem AIDS, chemoterapie, transplantace orgánů).
Candida albicans je typickým druhem střevní a ústní mikroflóry. Za normálních okolností u 80 % populace neškodí. Když se však nadmíru přemnoží, způsobuje kandidózu. Kandidóza je často diagnostikována osobám se sníženou imunitou. C. albicans se však může přemnožit i v krvi a v genitálním traktu. U dětí způsobuje rovněž nemoc zvanou moučnice či moučnivka. Při infekci se do těla dostává obvyklá jednobuněčná kvasinková forma. Ta se však vlivem prostředí může změnit v invazivní mnohobuněčnou vláknitou formu.
Moučnivka neboli orální kandidóza se může vyskytovat ve formě
Často se objevuje i u lidí se zubní náhradou, přičemž může docházet současně k postižení koutků. Moučnivka se objevuje i u novorozených dětí (asi tak desátý den po porodu). Přenáší se z těla matky, která byla postižena před porodem nevyléčenou vaginální mykózou. Riziko onemocnění moučnivkou se zvyšuje u předčasně narozených dětí, které mají nedostatečně rozvinutý imunitní systém.[1]
Zajímavou vlastností Candida albicans je nepravidelná délka a struktura jejích chromozomů. Příčinou je polymorfismus délky chromozomů, translokace některých částí, chybějící nebo naopak přebývající chromozomy (např. trizomie). Tyto změny chromatinu vedou k odchylkám v celkovém vzhledu, což je výhodnou evoluční strategií těchto patogenních hub (brání se tak proti imunitní odpovědi organismu).
Sekvenování genomu C. albicans významně změnilo způsob jejího současného výzkumu. Dále byly díky němu identifikovány nové taxony s odchylkami v genetickém kódu: Candida glabrata, C. dubliniensis, C. parapsilosis, C. guilliermondii, C. lusitaniae a C. tropicalis. Genom Candida albicans se rovněž srovnává s nepatogenními vřeckovýtrusnými houbami, čímž se zjišťuje, proč C. albicans přechází z komenzalického na parazitický způsob života.
V procesu, který na první pohled připomíná fenotypický dimorfismus, C. albicans střídá fenotypy včetně změny vzhledu buněk. Výzkum části DNA zvané WO-1 ukázal, že existují dva fenotypy. První forma roste jako bílá hladká kolonie, druhá je šlahounovitá a vytváří ploché šedé kolonie. Rovněž část 3153A vytváří nejméně sedm různých typů, které se liší vzhledem. U obou se přepínání fenotypů děje vratně a poměrně zřídka. Fenotypy jsou z generace na generaci dědičné.
V části 3153A byl nalezen gen SIR2 (Silent Information Regulator), který je pravděpodobně zodpovědný za přepínání fenotypů. SIR2 byl původně nalezen u Saccharomyces cerevisiae (pivní kvasinka), u níž je zodpovědný za utlumování exprese okolních genů. U kvasinek (a pravděpodobně i u Candida albicans) se tyto geny nacházejí právě nedaleko genů, které jsou zodpovědné za rozmnožování.
Dalším rozhodujícím faktorem je molekula Efg1p, která se podílí na střídání fenotypů. Efg1p je aktivní pouze u bílého buněčného cyklu, nikoliv u šedého. Nadmíru vysoká exprese tohoto genu způsobuje rychlou změnu šedého fenotypu na bílý. Ještě není zcela jasno, zda se dimorfismus neděje jako reakce na změnu v prostředí.
Při oslabení obranného systému se houbová infekce šíří do celého organizmu a napadá vnitřní orgány. V tomto stavu oslabení se z neškodné kvasinky stává agresivní a nebezpečná parazitická houba. V počáteční fázi se infekce skrývá pod maskou jiných onemocnění, která lékařská věda považuje za samostatné choroby. Příznaky mohou mít značně rozdílné formy a všechny zde není možno uvést.[2]
Klasická medicína houbovou infekci léčí nystatinem, fluconazolem či itraconazolem.[3] Pokud však pacient nezmění stravovací návyky (spočívající zejména v eliminaci jednoduchých cukrů) a nezlepší se fungování jeho imunitního systému, je námaha zbytečná a brzy dojde k recidivě nemoci.[2] MUDr. Petr Lukeš doporučuje neuskladňovat pečivo v igelitu (mikrotenu), resp. nejíst ho, protože již po třech hodinách na pečivu začíná růst plíseň Candida albicans.[4]
Candida albicans je druh kvasinky, jenž někdy u lidí způsobuje ústní a genitální houbové infekce. Tato mykóza je zejména častou příčinou nemocí a úmrtí u osob se sníženou imunitou (vlivem AIDS, chemoterapie, transplantace orgánů).
Candida albicans er en gærsvamp i slægten Candida. Under normale omstændigheder lever den i 80% af den menneskelige population uden at volde skade. Overvækst resulterer dog i sygdom og kaldes candidiasis.
Hyppige infektioner der er forårsaget af Candida albicans omfatter trøske og vaginitis.
Hos immunkompromiterede individer, f.eks. hos AIDS-patienter eller kræftpatienter, kan den sprede sig og give alvorlige infektioner.
Candida albicans er en gærsvamp i slægten Candida. Under normale omstændigheder lever den i 80% af den menneskelige population uden at volde skade. Overvækst resulterer dog i sygdom og kaldes candidiasis.
Hyppige infektioner der er forårsaget af Candida albicans omfatter trøske og vaginitis.
Hos immunkompromiterede individer, f.eks. hos AIDS-patienter eller kræftpatienter, kan den sprede sig og give alvorlige infektioner.
Candida albicans (früher Monilia albicans und Oidium albicans), der „Soorpilz“, ist ein Pilz der Candidagruppe, die den Hefepilzen zugeordnet wird. Er ist der häufigste Erreger der Kandidose (auch Candidose, Candidiasis, Candidamykose, Monoliasis, Soor oder bei Babys „Windelpilz“ genannt). Dieser Pilz ist bei Gleichwarmen („Warmblüter“) und somit auch beim Menschen häufig auf den Schleimhäuten von Mund und Rachen und im Genitalbereich sowie im Verdauungstrakt zu finden. Bei etwa 75 % aller gesunden Menschen kann er laut der Deutschen Gesellschaft für Ernährung nachgewiesen werden. Der Pilz kann auch zwischen Fingern und Zehen sowie auf den Finger- und Fußnägeln vorkommen.
C. albicans gehört zu den fakultativ pathogenen Erregern (nur unter bestimmten Bedingungen eine Krankheit auslösend) und ist als ein Saprobiont anzusehen, der in einem Gleichgewichtszustand mit der menschlichen Immunabwehr und anderen Mikroorganismen siedelt. Die Besiedelung durch diesen Pilz verursacht in der Regel kaum Beschwerden. Bei fehlender oder verminderter Immunität (im Rahmen von anderen Grundkrankheiten, wie Diabetes mellitus, Krebs, AIDS, oder durch die Gabe bestimmter Medikamente wie Antibiotika) kann die Besiedelung mit Candida albicans oder ihm verwandten Pilzen jedoch stark zunehmen, die sich dann als Mykose manifestiert. Meistens handelt es sich dabei um endogene Infektionen, das heißt, der Erreger war bereits vor Krankheitsausbruch am Ort der Infektion, seltener um exogene Infektionen, also durch von außen erworbene Erreger.
Behandeln lässt sich eine Candidose mit Antimykotika (Antipilzmittel), die in die Synthese der Pilzzellwand (zum Beispiel Caspofungin) oder der Zellmembran (zum Beispiel Fluconazol, Nystatin) eingreifen.
Candida lässt sich gut unter Zugabe von Antibiotika (zur Unterdrückung von Bakterienkolonien) auf einfachen Nährböden anzüchten und bildet in Kultur bei 37 °C innerhalb von ein bis zwei Tagen kleine weißliche Kolonien.
Candida ist ein polymorpher Pilz, d. h., er bildet unterschiedliche Wachstumsformen aus. Die einzelnen Pilzzellen sind rundlich-oval und haben einen Durchmesser von ungefähr 4–10 µm (extreme Werte aus der Literatur). Typisch sind für Candida albicans sowohl die Bildung von Pseudomyzelen (Fadenform) als auch die Bildung von echten Hyphen, die jedoch schon ein Hinweis für die nicht mehr saprobiotische, sondern invasive Besiedelungsform im Rahmen einer manifesten Infektion sind. Einzelne Myzelfäden können bereits mit bloßem Auge im Untersuchungsmaterial erkannt werden.
Candida bildet sogenannte Blastokonidien, die durch Sprossung entstehen (vgl. Konidiogenese und Dehiszenz). Auch Dauersporen, die sogenannten Chlamydosporen, sind ein wichtiges Unterscheidungsmerkmal von Candida albicans zu anderen Hefen, kommen jedoch auch bei einem engen und ebenfalls klinisch relevanten Verwandten, Candida dubliniensis, vor. Diese Chlamydosporen bilden eine widerstandsfähige Zellwand und sind größer als Blastokonidien. Candida albicans besitzt als diploider Organismus ein Genom mit einer Größe von 2x16 Megabasenpaaren, welches auf 2x8 Chromosomen verteilt ist. Lange war bei diesem Pilz kein sexuelles Stadium bekannt, so dass er zu den Fungi imperfecti zählte. Neuere Forschungsergebnisse deuten jedoch darauf hin, dass Candida albicans sich unter bestimmten Bedingungen sexueller Mechanismen für den Austausch genetischen Materials zwischen verschiedenen Isolaten bedient, und dass diese Fähigkeit eine Rolle in der Anpassung des Pilzes an bestimmte Stressbedingungen spielt.[1]
Seit 2013 ist bekannt, dass C. albicans vorübergehend in einer haploiden Form vorkommt, die sich durch sexuelle Fortpflanzung oder Autodiploidisierung in die diploide Form zurückverwandeln kann.[2]
Das KEGG-Genom von C. albicans besteht aus 14.629 proteincodierenden Genen und 32 RNA-kodierenden Genen.[3] 13 weitere Genome sind erfasst.[4] Die Proteinstruktur ist online verfügbar.[5]
Die Zahnkaries ist eine Erkrankung der Zahnhartgewebe Zahnschmelz und Dentin. Neuere Forschung hat ergeben, dass ein Zusammenspiel des kariogenen Streptococcus mutans mit dem Pilz Candida albicans besteht, wodurch das Bakterium seine Virulenz verändert. Der Pilz produziert Signalmoleküle, die Gene des Bakteriums zur Produktion zelleigener Antibiotika anregen. Das Bakterium kann durch den Pilz fremdes Erbgut aufnehmen. Streptococcus mutans bildet Dextrane, die zur Bildung der Plaque (Zahnbelag) beitragen. Mikroorganismen verfügen an ihrer Zellwand über spezielle Rezeptoren, die ihnen diese Bindung ermöglichen. Die Produktion klebriger Substanzen, einer wichtigen Voraussetzung für die Haftung des Streptococcus mutans auf dem Zahn, wird durch den Pilz unterstützt.[6]
Der Hefepilz Candida albicans steht mit vielen anderen Mikroben des humanen Mikrobioms in Symbiose, denn er trägt mit diesen zur Biofilmbildung bzw. regt sie zur Bildung eines Biofilms an.[7][8][9] Da Immunzellen nur bedingt gegen einen Biofilm vorgehen können, kann es auch bei immunkompetenten Patienten z. B. zu chronischen Reizungen, leichten chronischen Entzündungen, Gelenkschmerzen oder chronischer Müdigkeit kommen.[10] Zudem können temporär verabreichte, immunsuppressive Medikamente (z. B. Steroide, Kortison) oder Infektionen (Influenza) zur invasiven Ausbreitung des Biofilms beitragen. Dies kann zu chronischen Reizungen und Entzündungen im Bereich der inneren Organe (Endokarditis, Meningitis, Endophthalmitis etc.) führen.[11]
Der Biofilm begünstigt das Austauschen von Resistenzgenen zwischen einzelnen Bakterien.[12] Des Weiteren produziert der Hefepilz Candida albicans ein Sekret, welches undurchlässig für das Antibiotikum Vancomycin ist und somit die sich im Biofilm befindlichen Bakterien vor jenem Antibiotikum schützt.[7] Es ist davon auszugehen, dass auch andere Antibiotika von dem Sekret blockiert werden können. Das Sekret kann jedoch mit Antimykotika (z. B. Amphotericin-B, Anidulafungin) und einigen NSAR (z. B. Ibuprofen und Aspirin) gelöst werden.[7][13][14] Auch kann Stickstoffmonoxid bereits in einer für den Menschen ungefährlichen Konzentration bestimmte Mikroorganismen zur Auflösung des Biofilms anregen.[15]
Candida albicans (früher Monilia albicans und Oidium albicans), der „Soorpilz“, ist ein Pilz der Candidagruppe, die den Hefepilzen zugeordnet wird. Er ist der häufigste Erreger der Kandidose (auch Candidose, Candidiasis, Candidamykose, Monoliasis, Soor oder bei Babys „Windelpilz“ genannt). Dieser Pilz ist bei Gleichwarmen („Warmblüter“) und somit auch beim Menschen häufig auf den Schleimhäuten von Mund und Rachen und im Genitalbereich sowie im Verdauungstrakt zu finden. Bei etwa 75 % aller gesunden Menschen kann er laut der Deutschen Gesellschaft für Ernährung nachgewiesen werden. Der Pilz kann auch zwischen Fingern und Zehen sowie auf den Finger- und Fußnägeln vorkommen.
C. albicans gehört zu den fakultativ pathogenen Erregern (nur unter bestimmten Bedingungen eine Krankheit auslösend) und ist als ein Saprobiont anzusehen, der in einem Gleichgewichtszustand mit der menschlichen Immunabwehr und anderen Mikroorganismen siedelt. Die Besiedelung durch diesen Pilz verursacht in der Regel kaum Beschwerden. Bei fehlender oder verminderter Immunität (im Rahmen von anderen Grundkrankheiten, wie Diabetes mellitus, Krebs, AIDS, oder durch die Gabe bestimmter Medikamente wie Antibiotika) kann die Besiedelung mit Candida albicans oder ihm verwandten Pilzen jedoch stark zunehmen, die sich dann als Mykose manifestiert. Meistens handelt es sich dabei um endogene Infektionen, das heißt, der Erreger war bereits vor Krankheitsausbruch am Ort der Infektion, seltener um exogene Infektionen, also durch von außen erworbene Erreger.
Behandeln lässt sich eine Candidose mit Antimykotika (Antipilzmittel), die in die Synthese der Pilzzellwand (zum Beispiel Caspofungin) oder der Zellmembran (zum Beispiel Fluconazol, Nystatin) eingreifen.
Candida lässt sich gut unter Zugabe von Antibiotika (zur Unterdrückung von Bakterienkolonien) auf einfachen Nährböden anzüchten und bildet in Kultur bei 37 °C innerhalb von ein bis zwei Tagen kleine weißliche Kolonien.
Candida albicans je diploidna gljiva (oblik kvasca) koje se razmnožava seksualnim putem ali ne dolazi do mejoze. Dovodi do nastanka oportunističkih oralnih i genitalnih infekcija kod ljudi. Sistemne gljivične infekcije su značajne kod imunokompronitiranih pacijenata (AIDS, pacijenti na hemoterapiji, ili transplantirani pacijenti). Oboljenja koje uzrokuje candida se mogu javiti i kod pretjerane primjene antibiotika, kao i kod neadekvatne prehrane (visok unos ugljikohidrata). C. albicans je komenzal i nalazi se u normalnoj flori usne šupljine. Normalno se C. albicans može nalaziti kao dio fiziološke flore kod 80% humane populacije, bez štetnih efekata a njen prekomjeran rast i razmnožavanje se manifestira pojavom kandidijaze. Kandidijaza može zahvatiti genitalni trakt, probavni trakt ili se može naći u krvi.
Candida albicans je diploidna gljiva (oblik kvasca) koje se razmnožava seksualnim putem ali ne dolazi do mejoze. Dovodi do nastanka oportunističkih oralnih i genitalnih infekcija kod ljudi. Sistemne gljivične infekcije su značajne kod imunokompronitiranih pacijenata (AIDS, pacijenti na hemoterapiji, ili transplantirani pacijenti). Oboljenja koje uzrokuje candida se mogu javiti i kod pretjerane primjene antibiotika, kao i kod neadekvatne prehrane (visok unos ugljikohidrata). C. albicans je komenzal i nalazi se u normalnoj flori usne šupljine. Normalno se C. albicans može nalaziti kao dio fiziološke flore kod 80% humane populacije, bez štetnih efekata a njen prekomjeran rast i razmnožavanje se manifestira pojavom kandidijaze. Kandidijaza može zahvatiti genitalni trakt, probavni trakt ili se može naći u krvi.
Candida albicans is an opportunistic pathogenic yeast[5] that is a common member of the human gut flora. It can also survive outside the human body.[6][7] It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults.[8][9] It is usually a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions.[9][10] It is one of the few species of the genus Candida that causes the human infection candidiasis, which results from an overgrowth of the fungus.[9][10] Candidiasis is, for example, often observed in HIV-infected patients.[11] C. albicans is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue.[12][13] C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata are together responsible for 50–90% of all cases of candidiasis in humans.[10][14][15] A mortality rate of 40% has been reported for patients with systemic candidiasis due to C. albicans.[16] By one estimate, invasive candidiasis contracted in a hospital causes 2,800 to 11,200 deaths yearly in the US.[14] Nevertheless, these numbers may not truly reflect the true extent of damage this organism causes, given new studies indicating that C. albicans can cross the blood–brain barrier in mice.[17][18]
C. albicans is commonly used as a model organism for fungal pathogens.[19] It is generally referred to as a dimorphic fungus since it grows both as yeast and filamentous cells. However, it has several different morphological phenotypes including opaque, GUT, and pseudohyphal forms.[20][21] C. albicans was for a long time considered an obligate diploid organism without a haploid stage. This is, however, not the case. Next to a haploid stage C. albicans can also exist in a tetraploid stage. The latter is formed when diploid C. albicans cells mate when they are in the opaque form.[22] The diploid genome size is approximately 29 Mb, and up to 70% of the protein coding genes have not yet been characterized.[23] C. albicans is easily cultured in the lab and can be studied both in vivo and in vitro. Depending on the media different studies can be done as the media influences the morphological state of C. albicans. A special type of medium is CHROMagar Candida, which can be used to identify different Candida species.[24][25]
Candida albicans can be seen as a tautology. Candida comes from the Latin word candidus, meaning white. Albicans itself is the present participle of the Latin word albicō, meaning becoming white. This leads to white becoming white, making it a tautology.
It is often shortly referred to as thrush, candidiasis, or candida. More than a hundred synonyms have been used to describe C. albicans.[2][26] Over 200 species have been described within the candida genus. The oldest reference to thrush, most likely caused by C. albicans, dates back to 400 BCE in Hippocrates' work Of the Epidemics describing oral candidiasis.[2][27]
The genome of C. albicans is almost 16Mb for the haploid size (28Mb for the diploid stage) and consists of 8 sets of chromosome pairs called chr1A, chr2A, chr3A, chr4A, chr5A, chr6A, chr7A and chrRA. The second set (C. albicans is diploid) has similar names but with a B at the end. Chr1B, chr2B, ... and chrRB. The whole genome contains 6,198 open reading frames (ORFs). Seventy percent of these ORFs have not yet been characterized. The whole genome has been sequenced making it one of the first fungi to be completely sequenced (next to Saccharomyces cerevisiae and Schizosaccharomyces pombe).[11][23] All open reading frames (ORFs) are also available in Gateway-adapted vectors. Next to this ORFeome there is also the availability of a GRACE (gene replacement and conditional expression) library to study essential genes in the genome of C. albicans.[28][29] The most commonly used strains to study C. albicans are the WO-1 and SC5314 strains. The WO-1 strain is known to switch between white-opaque form with higher frequency while the SC5314 strain is the strain used for gene sequence reference.[30]
One of the most important features of the C. albicans genome is the high heterozygosity. At the base of this heterozygosity lies the occurrence of numeric and structural chromosomal rearrangements and changes as means of generating genetic diversity by chromosome length polymorphisms (contraction/expansion of repeats), reciprocal translocations, chromosome deletions, Nonsynonymous single-nucleotide polymorphisms and trisomy of individual chromosomes. These karyotypic alterations lead to changes in the phenotype, which is an adaptation strategy of this fungus. These mechanisms are further being explored with the availability of the complete analysis of the C. albicans genome.[31][32][33]
An unusual feature of the genus Candida is that in many of its species (including C. albicans and C. tropicalis, but not, for instance, C. glabrata) the CUG codon, which normally specifies leucine, specifies serine in these species. This is an unusual example of a departure from the standard genetic code, and most such departures are in start codons or, for eukaryotes, mitochondrial genetic codes.[34][35][36] This alteration may, in some environments, help these Candida species by inducing a permanent stress response, a more generalized form of the heat shock response.[37] However, this different codon usage makes it more difficult to study C. albicans protein-protein interactions in the model organism S. cerevisiae. To overcome this problem a C. albicans specific two-hybrid system was developed.[38]
The genome of C. albicans is highly dynamic, contributed by the different CUG translation, and this variability has been used advantageously for molecular epidemiological studies and population studies in this species. The genome sequence has allowed for identifying the presence of a parasexual cycle (no detected meiotic division) in C. albicans.[39] This study of the evolution of sexual reproduction in six Candida species found recent losses in components of the major meiotic crossover-formation pathway, but retention of a minor pathway.[39] The authors suggested that if Candida species undergo meiosis it is with reduced machinery, or different machinery, and indicated that unrecognized meiotic cycles may exist in many species. In another evolutionary study, introduction of partial CUG identity redefinition (from Candida species) into Saccharomyces cerevisiae clones caused a stress response that negatively affected sexual reproduction. This CUG identity redefinition, occurring in ancestors of Candida species, was thought to lock these species into a diploid or polyploid state with possible blockage of sexual reproduction.[40]
C. albicans exhibits a wide range of morphological phenotypes due to phenotypic switching and bud to hypha transition. The yeast-to-hyphae transition (filamentation) is a rapid process and induced by environmental factors. Phenotypic switching is spontaneous, happens at lower rates and in certain strains up to seven different phenotypes are known. The best studied switching mechanism is the white to opaque switching (an epigenetic process). Other systems have been described as well. Two systems (the high-frequency switching system and white to opaque switching) were discover by David R. Soll and colleagues.[41][42] Switching in C. albicans is often, but not always, influenced by environmental conditions such as the level of CO2, anaerobic conditions, medium used and temperature.[43] In its yeast form C. albicans ranges from 10 to 12 microns.[44] Spores can form on the pseudohyphae called chlamydospores which survive when put in unfavorable conditions such as dry or hot seasons.[45]
Although often referred to as dimorphic, C. albicans is, in fact, polyphenic (often also referred to as pleomorphic).[46] When cultured in standard yeast laboratory medium, C. albicans grows as ovoid "yeast" cells. However, mild environmental changes in temperature, CO2, nutrients and pH can result in a morphological shift to filamentous growth.[47][48] Filamentous cells share many similarities with yeast cells. Both cell types seem to play a specific, distinctive role in the survival and pathogenicity of C. albicans. Yeast cells seem to be better suited for the dissemination in the bloodstream while hyphal cells have been proposed as a virulence factor. Hyphal cells are invasive and speculated to be important for tissue penetration, colonization of organs and surviving plus escaping macrophages.[49][50][51] The transition from yeast to hyphal cells is termed to be one of the key factors in the virulence of C. albicans; however, it is not deemed necessary.[52] When C. albicans cells are grown in a medium that mimics the physiological environment of a human host, they grow as filamentous cells (both true hyphae and pseudohyphae). C. albicans can also form chlamydospores, the function of which remains unknown, but it is speculated they play a role in surviving harsh environments as they are most often formed under unfavorable conditions.[53]
The cAMP-PKA signaling cascade is crucial for the morphogenesis and an important transcriptional regulator for the switch from yeast like cells to filamentous cells is EFG1.[54][55]
Besides the well-studied yeast-to-hyphae transition other switching systems have been described.[56] One such system is the "high-frequency switching" system. During this switching different cellular morphologies (phenotypes) are generated spontaneously. This type of switching does not occur en masse, represents a variability system and it happens independently from environmental conditions.[43] The strain 3153A produces at least seven different colony morphologies.[57][42][58] In many strains the different phases convert spontaneously to the other(s) at a low frequency. The switching is reversible, and colony type can be inherited from one generation to another. Being able to switch through so many different (morphological) phenotypes makes C. albicans able to grow in different environments, both as a commensal and as a pathogen.[59]
In the 3153A strain, a gene called SIR2 (for silent information regulator), which seems to be important for phenotypic switching, has been found.[60][61] SIR2 was originally found in Saccharomyces cerevisiae (brewer's yeast), where it is involved in chromosomal silencing—a form of transcriptional regulation, in which regions of the genome are reversibly inactivated by changes in chromatin structure (chromatin is the complex of DNA and proteins that make chromosomes). In yeast, genes involved in the control of mating type are found in these silent regions, and SIR2 represses their expression by maintaining a silent-competent chromatin structure in this region.[62] The discovery of a C. albicans SIR2 implicated in phenotypic switching suggests it, too, has silent regions controlled by SIR2, in which the phenotype-specific genes may reside. How SIR2 itself is regulated in S. cerevisiae may yet provide more clues as to the switching mechanisms of C. albicans.
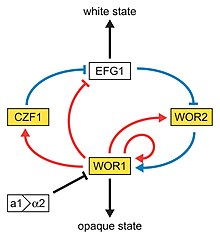
Next to the dimorphism and the first described high-frequency switching system C. albicans undergoes another high-frequency switching process called white to opaque switching, which is another phenotypic switching process in C. albicans. It was the second high-frequency switching system discovered in C. albicans.[41] The white to opaque switching is an epigenetic switching system.[63] Phenotypic switching is often used to refer to white-opaque switching, which consists of two phases: one that grows as round cells in smooth, white colonies (referred to as white form) and one that is rod-like and grows as flat, gray colonies (called opaque form). This switch from white cells to opaque cells is important for the virulence and the mating process of C. albicans as the opaque form is the mating competent form, being a million times more efficient in mating compared to the white type.[63][64][65] This switching between white and opaque form is regulated by the WOR1 regulator (White to Opaque Regulator 1) which is controlled by the mating type locus (MTL) repressor (a1-α2) that inhibits the expression of WOR1.[66] Besides the white and opaque phase there is also a third one: the gray phenotype. This phenotype shows the highest ability to cause cutaneous infections. The white, opaque and gray phenotypes form a tristable phenotypic switching system. Since it is often difficult to differentiate between white, opaque and gray cells phloxine B, a dye, can be added to the medium.[59]
A potential regulatory molecule in the white to opaque switching is Efg1p, a transcription factor found in the WO-1 strain that regulates dimorphism, and more recently has been suggested to help regulate phenotypic switching. Efg1p is expressed only in the white and not in the gray cell-type, and overexpression of Efg1p in the gray form causes a rapid conversion to the white form.[67][68]
Glucose starvation is a likely common environmental stress encountered by C. albicans in its natural habitat.[69] Glucose starvation causes an increase in intracellular reactive oxygen. This stress can lead to mating between two individuals of the same mating type, an interaction that may be frequent in nature under stressful conditions.[69]
A very special type of phenotypic switch is the white-GUT switch (Gastrointestinally-IndUced Transition). GUT cells are extremely adapted to survival in the digestive tract by metabolic adaptations to available nutrients in the digestive tract. The GUT cells live as commensal organisms and outcompete other phenotypes. The transition from white to GUT cells is driven by passage through the gut where environmental parameters trigger this transition by increasing the WOR1 expression.[70][71]
Candida is found worldwide but most commonly compromises immunocompromised individuals diagnosed with serious diseases such as HIV and cancer. Candida are ranked as one of the most common groups of organisms that cause hospital-acquired infections. Especially high-risk individuals are patients that have recently undergone surgery, a transplant or are in the Intensive Care Units (ICU),[72] C. albicans infections is the top source of fungal infections in critically ill or otherwise immunocompromised patients.[73] These patients predominantly develop oropharyngeal or thrush candidiasis, which can lead to malnutrition and interfere with the absorption of medication.[74] Methods of transmission include mother to infant through childbirth, people-to-people acquired infections that most commonly occur in hospital settings where immunocompromised patients acquire the yeast from healthcare workers and has a 40% incident rate. People can become infected after having sex with a woman that has an existing vaginal yeast infection.[72] Parts of the body that are commonly infected include the skin, genitals, throat, mouth, and blood.[75] Distinguishing features of vaginal infection include discharge, and dry and red appearance of vaginal mucosa or skin. Candida continues to be the fourth most commonly isolated organism in bloodstream infections.[76] Healthy people usually do not suffer (severely) from superficial infections caused by a local alteration in cellular immunity as seen by asthma patients that use oral corticosteroids.
It commonly occurs as a superficial infection on mucous membranes in the mouth or vagina. Once in their lives around 75% of women will suffer from vulvovaginal candidiasis (VVC) and about 90% of these infections are caused by C. albicans. It may also affect a number of other regions. For example, higher prevalence of colonization of C. albicans was reported in young individuals with tongue piercing, in comparison to unpierced matched individuals,[77] but not in healthy young individuals who use intraoral orthodontic acrylic appliances.[78] To infect host tissue, the usual unicellular yeast-like form of C. albicans reacts to environmental cues and switches into an invasive, multicellular filamentous form, a phenomenon called dimorphism.[79] In addition, an overgrowth infection is considered a superinfection, the term usually applied when an infection becomes opportunistic and very resistant to antifungals. It then becomes suppressible by antibiotics. The infection is prolonged when the original sensitive strain is replaced by the antibiotic-resistant strain.[80]
Candidiasis is known to cause gastrointestinal (GI) symptoms particularly in immunocompromised patients or those receiving steroids (e.g. to treat asthma) or antibiotics. Recently, there is an emerging literature that an overgrowth of fungus in the small intestine of non-immunocompromised subjects may cause unexplained GI symptoms. Small intestinal fungal overgrowth (SIFO) is characterized by the presence of an excessive number of fungal organisms in the small intestine associated with gastrointestinal symptoms. The most common symptoms observed in these patients were belching, bloating, indigestion, nausea, diarrhea, and gas. The underlying mechanism(s) that predisposes to SIFO is unclear. Further studies are needed; both to confirm these observations and to examine the clinical relevance of fungal overgrowth.[9][10][81]
Systemic fungal infections (fungemias) including those by C. albicans have emerged as important causes of morbidity and mortality in immunocompromised patients (e.g., AIDS, cancer chemotherapy, organ or bone marrow transplantation). C. albicans often forms biofilms inside the body. Such C. albicans biofilms may form on the surface of implantable medical devices or organs. In these biofilms it is often found together with Staphylococcus aureus.[12][13][82][83] Such multispecies infections lead to higher mortalities.[84] In addition hospital-acquired infections by C. albicans have become a cause of major health concerns.[11][85] Especially once candida cells are introduced in the bloodstream a high mortality, up to 40–60% can occur.[11][86]
Although Candida albicans is the most common cause of candidemia, there has been a decrease in the incidence and an increased isolation of non-albicans species of Candida in recent years.[87] Preventive measures include maintaining a good oral hygiene, keeping a healthy lifestyle including good nutrition, the careful use of antibiotics, treatment of infected areas and keeping skin dry and clean, free from open wounds.[88][89]
The link between C. albicans and Crohn's disease has been investigated in a large cohort. This study demonstrated that members of families with multiple cases of Crohn's disease were more likely to be colonized by C. albicans than members of control families.[90] Experimental studies show that chemically induced colitis promotes C. albicans colonization. In turn, C. albicans colonization generates anti-Saccharomyces cerevisiae antibodies (ASCA), increases inflammation, histological scores and pro-inflammatory cytokine expression.[91][92]
There are relatively few drugs that can successfully treat Candidiasis.[93][94] Treatment commonly includes:[95]
Similarly to antibiotic resistance, resistance to many anti-fungals is becoming a problem. New anti-fungals have to be developed to cope with this problem since only a limited number of anti-fungals are available.[93][97] A general problem is that in contrast to bacteria, fungi are often overlooked as a potential health problem.[98]
Given the fact that candidiasis is the fourth- (to third-) most frequent hospital acquired infection worldwide it leads to immense financial implications. Approximately 60,000 cases of systemic candidiasis each year in the USA alone lead up to a cost to be between $2–4 billion.[99] The total costs for candidiasis are among the highest compared to other fungal infections due to the high prevalence.[100] The immense costs are partly explained by a longer stay in the intensive care unit or hospital in general. An extended stay for up to 21 more days compared to non-infected patients is not uncommon.[101]
Gasdermin D (GSDMD) is a protein that in humans is encoded by the GSDMD gene and is a known target of the inflammasome and acts as an effector molecule of programmed cell death known as pyroptosis. This protein determines cell lysis to prevent pathogen replication and results in the release of the inflammatory cytokine interleukin-1β (IL-1β) into the extracellular space to recruit and activate immune cells at the site of infection. Inflammasome activation due to C.albicans infection triggers the release of a cytokine storm necessary to fight the pathogen. Excessive release of these pro-inflammatory mediators has been shown to exaggerate systemic inflammation leading to vascular injury and damage to vital organs. Unfortunately, Candida albicans therapy is often ineffective despite the availability of many antifungal drugs, mainly because of resistance phenomena. During conventional pyroptosis controlled by the inflammasome-GSDMD axis is hijacked by C. albicans to facilitate escape from macrophages through unfolding of hyphae and candidalysin, a fungal toxin released from hyphae. It has been shown[102] that disruption of GSDMD in macrophages infected with Candida albicans reduces the fungal load. In addition, the presence of hyphae and candidalysin are key factors in the activation of GSDMD and the release of Candida from macrophages. Also using Candida-infected mice, inhibition of GSDMD has been shown to paradoxically improve prognosis and survival, indicating that this protein may be a potential therapeutic target in C. albicans-induced sepsis.
The biofilm of C. albicans is formed in four steps. First, there is the initial adherence step, where the yeast-form cells adhere to the substrate. The second step is called Intermediate step, where the cells propagate to form microcolonies, and germ tubes form to yield hyphae. In the maturation step, the biofilm biomass expands, the extracellular matrix accumulates and drug resistance increases. In the last step of biofilm formation, the yeast-form cells are released to colonize the surrounding environment (dispersion). Yeast cells released from a biofilm have novel properties, including increased virulence and drug tolerance.[103][104][105]
Zap1, also known as Csr1 and Sur1 (zinc-responsive activator protein), is a transcription factor which is required for the hypha formation in C. albicans biofilms. Zap1 controls the equilibrium of yeast and hyphal cells, the zinc transporters and zinc regulated genes in biofilms of C. albicans.[106]
Zinc (Zn2+) is important for cell function of C. albicans and Zap1 controls the Zinc levels in the cells through the zinc transporters Zrt1 and Zrt2. The regulation of zinc concentration in the cells is important for the cell viability and if the zinc levels get too high, it is toxic for the cells. The Zrt1 is transporting the zinc ions with high affinity and the Zrt2 is transporting the zinc ions with low affinity.[107]
The ability to switch between yeast cells and hyphal cells is an important virulence factor. Many proteins play a role in this process. Filamentation in C. albicans is a very complex process.[108] The formation of hyphae can for example help Candida albicans to escape from macrophages in the human body.[109] Moreover, C. albicans undergo yeast-to-hyphal transition within the acidic macrophage phagosome. This initially causes phagosome membrane distension which eventually leads to phagosomal alkalinization by physical rupture, followed by escape.[110]
Hwp1 stands for Hyphal wall protein 1. Hwp1 is a mannoprotein located on the surface of the hyphae in the hyphal form of C. albicans. Hwp1 is a mammalian transglutaminase substrate. This host enzyme allows Candida albicans to attach stably to host epithelial cells.[111] Adhesion of C. albicans to host cells is an essential first step in the infection process for colonization and subsequent induction of mucosal infection.
The RNA-binding protein Slr1 plays a role in instigating hyphal formation and virulence in C. albicans.[112]
Candidalysin is a cytolytic 31-amino acid α-helical peptide toxin that is released by C. albicans during hyphal formation. It contributes to virulence during mucosal infections.[113]
Due to its nature as a model organism, being an important human pathogen and the alternative codon usage (CUG translated into serine rather than leucine), several specific projects and tools have been created to study C. albicans.[11] The diploid nature and the absence of a sexual cycle, however, makes it a hard to study organism. In the last 20 years, however, many systems have been developed to study C. albicans in a more in depth genetic level.[19]
The most used selection markers in C. albicans are the CaNAT1 resistance marker (confers resistance against nourseothricin) and MPAr or IMH3r (confers resistance to mycophenolic acid).[114] Next to the above-mentioned selection makers a few auxotrophic strains were generated to work with auxotrophic makers. The URA3 marker (URA3 blaster method) is an often-used strategy in uridine auxotrophic strains; however, studies have shown that differences in URA3 position in the genome can be involved in the pathogeny of C. albicans.[115] Besides the URA3 selection one can also use the histidine, leucine and arginine autotrophy. The advantage of using those autotrophies lies in the fact that they exhibit wild-type or nearly wild-type virulence in a mouse model compared to the URA3 system.[116] One application of the leucine, arginine and histidine autotrophy is for example the candida two-hybrid system.[38]
The full genome of C. albicans has been sequenced and made publicly available in a Candida database. The heterozygous diploid strain used for this full genome sequence project is the laboratory strain SC5314. The sequencing was done using a whole-genome shotgun approach.[117]
Every predicted ORF has been created in a gateway adapted vector (pDONR207) and made publicly available. The vectors (plasmids) can be propagated in E.coli and grown on LB+gentamicin medium. This way every ORF is readily available in an easy to use vector. Using the gateway system it is possible to transfer the ORF of interest to any other gateway adapted vector for further studies of the specific ORF.[29][118]
Contrary to the yeast S. cerevisiae episomal plasmids do not stay stable in C. albicans. In order to work with plasmids in C. albicans an integrative approach (plasmid integration into the genome) thus has to be used. A second problem is that most plasmid transformations are rather inefficient in C. albicans; however, the CIp10 plasmid overcomes these problems and can be used with ease to transform C. albicans in a very efficient way. The plasmid integrates inside the RP10 locus as disruption of one RP10 allele does not seem to affect the viability and growth of C. albicans. Several adaptations of this plasmid have been made after the original became available.[119][120]
Due to the aberrant codon usage of C. albicans it is less feasible to use the common host organism (Saccharomyces cerevisiae) for two-hybrid studies. To overcome this problem a C. albicans two-hybrid (C2H) system was created. The strain SN152 that is auxotrophic for leucine, arginine and histidine was used to create this C2H system. It was adapted by integrating a HIS1 reporter gene preceded by five LexAOp sequences. In the C2H system the bait plasmid (pC2HB) contains the Staphylococcus aureus LexA BD, while the prey plasmid (pC2HP) harbors the viral AD VP16. Both plasmids are integrative plasmids since episomal plasmids do not stay stable in C. albicans. The reporter gene used in the system is the HIS1 gene. When proteins interact, the cells will be able to grow on medium lacking histidine due to the activation of the HIS1 reporter gene.[11][38] Several interactions have thus far been detected using this system in a low scale set up.[38][121] A first high-throughput screening has also been performed.[122][123] Interacting proteins can be found at the BioGRID.[124]
Besides the C2H system, a BiFC system has been developed to study protein-protein interactions in C. albicans. With this systems protein interactions can be studied in their native sub cellular location contrary to a C2H system in which the proteins are forced into the nucleus. With BiFC one can study for example protein interactions that take place at the cell membrane or vacuolar membrane.[123][125][126]
Both DNA and protein microarrays were designed to study DNA expression profiles and antibody production in patients against C. albicans cell wall proteins.[120][127]
Using a tetracycline-regulatable promoter system a gene replacement and conditional expression (GRACE) library was created for 1,152 genes. By using the regulatable promoter and having deleted 1 of the alleles of the specific gene it was possible to discriminate between non-essential and essential genes. Of the tested 1,152 genes 567 showed to be essential. The knowledge on essential genes can be used to discover novel antifungals.[28]
CRISPR/Cas9 has been adapted to be used in C. albicans.[128] Several studies have been performed using this system.[129][130]
C. albicans has been used in combination with carbon nanotubes (CNT) to produce stable electrically conductive bio-nano-composite tissue materials that have been used as temperature-sensing elements.[131]
Candida albicans is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usually a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions. It is one of the few species of the genus Candida that causes the human infection candidiasis, which results from an overgrowth of the fungus. Candidiasis is, for example, often observed in HIV-infected patients. C. albicans is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue. C. albicans, C. tropicalis, C. parapsilosis, and C. glabrata are together responsible for 50–90% of all cases of candidiasis in humans. A mortality rate of 40% has been reported for patients with systemic candidiasis due to C. albicans. By one estimate, invasive candidiasis contracted in a hospital causes 2,800 to 11,200 deaths yearly in the US. Nevertheless, these numbers may not truly reflect the true extent of damage this organism causes, given new studies indicating that C. albicans can cross the blood–brain barrier in mice.
C. albicans is commonly used as a model organism for fungal pathogens. It is generally referred to as a dimorphic fungus since it grows both as yeast and filamentous cells. However, it has several different phenotypes including opaque, GUT, and pseudohyphal forms. C. albicans was for a long time considered an obligate diploid organism without a haploid stage. This is, however, not the case. Next to a haploid stage C. albicans can also exist in a tetraploid stage. The latter is formed when diploid C. albicans cells mate when they are in the opaque form. The diploid genome size is approximately 29 Mb, and up to 70% of the protein coding genes have not yet been characterized. C. albicans is easily cultured in the lab and can be studied both in vivo and in vitro. Depending on the media different studies can be done as the media influences the morphological state of C. albicans. A special type of medium is CHROMagar Candida, which can be used to identify different Candida species.
Candida albicans estas specio de gistofungoj. Ĝi povas kaŭzi kandidozon.
Candida albicans es un hongo diploide en forma levadura[2][3] de la familia de los sacaromicetos. Es un patógeno oportunista, ya que se comporta como un organismo comensal al formar parte la microbiota normal de los tractos respiratiorio, gastrointestinal y genitourinal, pero una disrupción en el estado imunológico del hospedador o en el entorno local lo convierten en patógeno.[4] Una de sus características más notables en relación con su patogenicidad es su versatilidad morfológica para cambiar entre su forma de levadura a hifas filamentosas.[5]
Tiene una función relevante en la digestión de los azúcares, mediante un proceso de fermentación.
Candida albicans puede asumir patogeneidad, provocando la candidiasis; en ese caso, se presenta como una afección vaginal (vaginitis), de la cavidad oral (afta), del intestino o de la piel. También puede provocar hongos vaginales.
Se ha investigado una posible relación entre la candidiasis y el cáncer, bien mediante la producción de micotoxinas o compuestos cancerígenos o mediante el desarrollo de inflamación crónica y procesos que interfieren con el ciclo vital de las células.[9]
Uno de los más interesantes hechos del genoma de C. albicans es la ocurrencia de rearreglos numéricos y estructurales cromosómicos, como medio de generar diversidad genética, dando longitudes de cromosomas con polimorfismo (contracción / expansión de repeticiones), translocaciones recíprocas, borrados cromosómicos y trisomía de cromosomas individuales. Estas alteraciones del cariotipo generan cambios en el fenotipo, que es una estrategia de adaptación de esta levadura. Estos mecanismos genéticos serán mejor interpretados con el análisis completo del genoma de C. albicans.
Su genoma en la "raza SC5314" fue secuenciado en el Centro de Tecnología y Secuenciación del ADN de Stanford.[10][11] El genoma de la cepa WO1 fue secuenciado por el Instituto Broad de MIT y de la Universidad Harvard.[12]
El secuenciado del genoma de C. albicans y subsecuentemente de los genomas de varias otras especies de Candida médicamente relevantes ha cambiado profundamente e irreversiblemente los modos de estudiar las especies de Candida.[3] El secuenciado genómico de C. albicans se lanzó en octubre de 1996. Y así, durante 10 años, culminando con el lanzamiento del agrupamiento del diploide 19, que provee una versión haploide del genoma, acompañado de datos de regiones alélicas en el genoma.[3] Otra refinada conjunción 20 con ocho ensambles de cromosomas de C. albicans se lanzó en el 2006. Se están desarrollando otras secuencias genómicas de Candida para: C. glabrata, C. dubliniensis, C. parapsilosis, C. guilliermondii, C. lusitaniae, y C. tropicalis.[3]
El genoma de C. albicans es altamente dinámico, y esta variabilidad ha sido usada ventajosamente en estudios epidemiológicos moleculares de C. albicans y estudios de población en esta especie. Un descubrimiento remarcable que ha sido llevado en la secuenciación del genoma es la presencia de un ciclo parasexual en C. albicans. Este ciclo parasexual está bajo el control de loci de masculinidad, y permuta entre fenotipos blanco y opaco. Investigando el rol que el proceso de apareamiento juega en la dinámica de las poblaciones de C. albicans o en otros aspectos de la biología y la patogeneidad de C. albicans representa un importante foco para futuros estudios.[3]
Según la extensión de la infección y el estado general del paciente, se decide un tratamiento tópico o sistémico. Así, tópicamente se puede emplear clotrimazol al 1 por ciento, miconazol, ketoconazol, sertoconazol, terbinafina o naftilina. Los tratamientos sistémicos más frecuentemente empleados son itraconazol o fluconazol. El pronóstico es bueno, y son curativos tanto los tratamientos tópicos como sistémicos. Sin embargo, si los factores predisponentes de estas micosis no se corrigen es posible otra nueva infección. Es, pues, necesario aplicar el tratamiento lo más pronto posible.
Candida albicans es un hongo diploide en forma levadura de la familia de los sacaromicetos. Es un patógeno oportunista, ya que se comporta como un organismo comensal al formar parte la microbiota normal de los tractos respiratiorio, gastrointestinal y genitourinal, pero una disrupción en el estado imunológico del hospedador o en el entorno local lo convierten en patógeno. Una de sus características más notables en relación con su patogenicidad es su versatilidad morfológica para cambiar entre su forma de levadura a hifas filamentosas.
Tiene una función relevante en la digestión de los azúcares, mediante un proceso de fermentación.
Candida albicans on seeneliik Candida perekonnast. Candida albicans võib mitmete tegurite toimel elusorganismides paljuneda ohtlikuks seenpatogeeniks.
Candida albicans'iga kommensalismis elavad organismid pole teada, uuringute kohaselt eluneb seen ühes biokiles vahel koos Candida auris'e ja bakteri Staphylococcus aureus'ega ja kuulub mitmete loomade, näiteks lindude, maoliste ja imetajate mikrofloorasse.
Candida albicans kuulub ka inimese normaalse naha-, limaskesta ja tupe mikrofloorasse, st elab inimese nahal ja limaskestadel; siin hoiavad tema vohamist kontrolli all teised normaalse mikrofloora mikroobid ning ka katteepiteeli toodetud antimikroobsed ained.
Candida albicans'i peetakse oportunistlikuks pärmseenhaiguse tekitajaks osadel, peamiselt immuunpuudulikkuse seisunditega eri vanuses inimestel (vastsündinutest vanuriteni).
Seen võib ületada teiste mikroobide elualad, kui antibiootikumide tarvitamise tagajärjel saavad kahjustada kasulike bakterite kolooniad või kui neil tekivad iseäranis soodsad kasvutingimused, näiteks põhjustada suhkurtõbe ja kandidoose.[3] Sattudes vereringesse võib seen mitmes elundis nagu süda, kopsud, põrn, maks jne kandideemiat, nahal aga nahapärmseentõbe, ja ka generaliseerunud kandidoosi (candidosis systemica) esile kutsuda. Nosokomiaalse fungeemia korral peetakse elumusprognoose halvaks.
Candida albicans kasvab ka mitmete seenevastaste ainete, näiteks asoolide, ehhinokandiinide ja flukonasooli juures.
Candida albicans on seeneliik Candida perekonnast. Candida albicans võib mitmete tegurite toimel elusorganismides paljuneda ohtlikuks seenpatogeeniks.
Candida albicans'iga kommensalismis elavad organismid pole teada, uuringute kohaselt eluneb seen ühes biokiles vahel koos Candida auris'e ja bakteri Staphylococcus aureus'ega ja kuulub mitmete loomade, näiteks lindude, maoliste ja imetajate mikrofloorasse.
Candida albicans kuulub ka inimese normaalse naha-, limaskesta ja tupe mikrofloorasse, st elab inimese nahal ja limaskestadel; siin hoiavad tema vohamist kontrolli all teised normaalse mikrofloora mikroobid ning ka katteepiteeli toodetud antimikroobsed ained.
Candida albicans'i peetakse oportunistlikuks pärmseenhaiguse tekitajaks osadel, peamiselt immuunpuudulikkuse seisunditega eri vanuses inimestel (vastsündinutest vanuriteni).
Seen võib ületada teiste mikroobide elualad, kui antibiootikumide tarvitamise tagajärjel saavad kahjustada kasulike bakterite kolooniad või kui neil tekivad iseäranis soodsad kasvutingimused, näiteks põhjustada suhkurtõbe ja kandidoose. Sattudes vereringesse võib seen mitmes elundis nagu süda, kopsud, põrn, maks jne kandideemiat, nahal aga nahapärmseentõbe, ja ka generaliseerunud kandidoosi (candidosis systemica) esile kutsuda. Nosokomiaalse fungeemia korral peetakse elumusprognoose halvaks.
Candida albicans kasvab ka mitmete seenevastaste ainete, näiteks asoolide, ehhinokandiinide ja flukonasooli juures.
Candida albicans Candida generoko onddo mikroskopikoa da, kandidiasi izeneko gaitza sortzen duena.
Onddo hau gure organismoaren biztanle komentsala da eta gorputzaren atal desberdinak kolonizatzen ditu inongo gaitzik sortu gabe: ahoa, heste lodia eta bagina, esaterako. Bertan, kopuru txikietan dago eta -egoera normaletan- ez du gaixotasunik eragiten.
Immunitate-sistema ahuldua duten gizabanakoengan (HIESa dutenak, minbizidunak, diabetes larria dutenak, transplantatuak...), bereziki, Candida albicansek kandidiasi eragin dezake. Ohikoa da ere gaitz hori agertzea haien flora bakteriarra desorekatuta duten pertsonengan (antibiotikoak luzaro hartu dutenengan, adibidez).
Candida albicans legamien taldeko onddoa da. Legamiak onddo mikroskopikoak dira, berezko ezaugarri morfologiko eta fisiologiko dituztenak.
Morfologiari dagokionez, Candida albicans polimorfikoa da, morfologia desberdinak ditu. Esaterako, infektatzen duenean morfologia haritsua du, baina laborategiko hazkuntza-inguruetan hazten denean zelulabakarra da, zelula isolatuetan agertu ohi da [1]. Era berean, zelula bakar horiek itxura desberdinak har ditzakete: biribilak, oboideak, etab.
Onddo honen andui batzuen genoma sekuentziatu da dagoeneko [2]. Eukariota izanik, onddoak 8 kromosoma-bikote ditu, eta 6400 gene inguru.
Nahiz eta patogeno hertsia ez izan, Candida albicans mikrobio oportunista da. Horrek esan nahi du gaitza (kandidiasia) sor dezakeela aldeko baldintzak dituenean.
Bi dira kandidiasia harrapatzeko joera handiagoak dituzten gaixoak:
Kandidiasiak gorputzeko hainbat atali eraso diezaioke: ahoari, digestio-aparatuari, baginari eta larruazalari.
Immunitate-sistema indartsua duten gizabanakoengan kandidiasia erraz sendatzen da, baina immunogutxituengan septizemia eta beste ondorio larri batzuk eragin ditzake. Azken kasu honetan tratamendua askoz zailagoa da.
Candida albicans Candida generoko onddo mikroskopikoa da, kandidiasi izeneko gaitza sortzen duena.
Onddo hau gure organismoaren biztanle komentsala da eta gorputzaren atal desberdinak kolonizatzen ditu inongo gaitzik sortu gabe: ahoa, heste lodia eta bagina, esaterako. Bertan, kopuru txikietan dago eta -egoera normaletan- ez du gaixotasunik eragiten.
Immunitate-sistema ahuldua duten gizabanakoengan (HIESa dutenak, minbizidunak, diabetes larria dutenak, transplantatuak...), bereziki, Candida albicansek kandidiasi eragin dezake. Ohikoa da ere gaitz hori agertzea haien flora bakteriarra desorekatuta duten pertsonengan (antibiotikoak luzaro hartu dutenengan, adibidez).
Candida albicans on Candida-sukuun kuuluva hiiva ja on osa terveen ihmisen normaalia mikrobistoa. C. albicans esiintyy muun muassa iholla, kielessä ja ruoansulatuskanavassa. Sitä kutsutaan myös opportunistiseksi patogeeniksi, koska se voi aiheuttaa hiivasienitulehduksen, mikäli elimistön normaalin mikrobiston tasapaino muuttuu esim. antibioottikuurin aikana.[1]
Candida albicans muodostaa biofilmin neljässä eri vaiheessa. Ensimmäinen vaihe on kiinnittymisvaihe, jossa hiivasolut kiinnittyvät valikoituun pintamateriaaliin. Välivaiheessa hiivasolut muodostavat hyphae soluja ja kypsymisvaiheessa biofilmin biomassa laajenee ja lääkkeiden kestävyys paranee. Viimeisessä vaiheessa biofilmi vapauttaa ympärilleen uusia hiivasoluja, joilla on vahvistuneempi virulenssi. Hiiva ja hyphae-solut ovat ratkaisevia biofilmin muodostumisen kannalta.[2]
Zap1, joka tunnetaan myös lyhenteillä Csr1 and Sur1, on sinkkiin reagoiva aktivaattoriproteiini. Zap1 on siis transkriptiotekijä, jonka olemassaoloa tarvitaan hypha-solujen muodostamiseen C. albicans biofilmin muodostuksessa. Zap1 myös kontrolloi hiiva ja hyphae-solujen tasapainoa, sinkin transportereita sekä geenejä, jotka säätelevät sinkin määrää C. albicans biofilmeissä.[3]
Sinkki (Zn2+) on tärkeä osa C. albicans solujen toiminnalle, jonka pitoisuutta Zap1 kontrolloi sinkin transporttereiden avulla. Näitä kyseisiä transporttereita ovat ZRT1 ja ZRT2, jotka kuuluvat ZIP-transportereihin. Sinkki-pitoisuuden säätely soluissa on tärkeää solujen elintoimintojen kannalta. Liika pitoisuus on myrkyllistä soluille. ZRT1 kuljettaa sinkkiä soluihin korkealla affiniteetilla ja ZRT2 matalalla affiniteetilla.[4]
Candida albicans on Candida-sukuun kuuluva hiiva ja on osa terveen ihmisen normaalia mikrobistoa. C. albicans esiintyy muun muassa iholla, kielessä ja ruoansulatuskanavassa. Sitä kutsutaan myös opportunistiseksi patogeeniksi, koska se voi aiheuttaa hiivasienitulehduksen, mikäli elimistön normaalin mikrobiston tasapaino muuttuu esim. antibioottikuurin aikana.
Candida albicans est l'espèce de levure la plus importante et la plus connue du genre Candida.
Candida albicans est un organisme vivant à l'état naturel dans les muqueuses de l'être humain. On le retrouve dans le tube digestif de 70% des adultes sains[1], et il n'entraîne habituellement aucune maladie ou symptôme en particulier. C'est un organisme commensal saprophyte.
Ce champignon pathogène provoque des infections fongiques (candidiase ou candidose) essentiellement au niveau des muqueuses digestive et gynécologique. Les candidoses sont une cause importante de mortalité chez les patients immunodéprimés comme les patients atteints du sida, les patients cancéreux sous chimiothérapie ou après transplantation de moelle osseuse. Les candidoses orale et œsophagienne sont fréquentes chez le patient atteint du sida. Lorsque Candida s'infiltre dans le flux sanguin, l'infection devient systémique et on parle alors de candidémie (ou candémie). Les candidémies sont caractérisées par une mortalité de l'ordre de 40 %. C. albicans peut donner également une multitude d'autres infections car il s'agit d'un pathogène opportuniste très polyvalent : il peut être responsable d'infection superficielle cutanée, causer un érythème fessier chez les nouveau-nés, une bronchopneumonie et, ou une pneumonie, une vaginite, une balanite ou être responsable d'infections profondes.
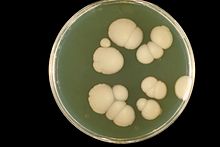
Au laboratoire médical, la culture en boîte de Petri des Candida donne des colonies qui sont grandes, rondes, de couleur blanche ou crème (albicans signifie « blanchâtre »).
Le fait que C. albicans soit classé comme étant un champignon asexué peut paraître surprenant, vu sa proximité phylogénétique avec des levures sexuées telles que Saccharomyces cerevisiae. De plus, des gènes impliqués dans le mating (en) et la méiose chez S. cerevisiae ont des orthologues chez C. albicans. La reproduction de C. albicans est majoritairement clonale, avec des échanges génétiques limités entre individus. Pourtant, la découverte de gènes de mating laisse à penser que C. albicans a gardé la capacité de se reproduire et de se recombiner. Ces gènes de mating, appelés MTL (mating type-like), possèdent de grandes similitudes avec les gènes MAT rencontrés chez S. cerevisiae, avec la différence notable que C. albicans possède 4 gènes MTL et non 3 comme c'est le cas chez S. cerevisiae. La plupart des souches de C. albicans sont hétérozygotes pour ces locus et seules 3 à 7 % des souches rencontrées dans la nature sont homozygotes. À l'inverse de S. cerevisiae, pour lesquelles toutes les cellules sont compétentes, seules les souches de C. albicans ayant subi un switch phénotypique de blanc à opaque sont compétentes. Il y a 2 connexions majeures entre le système de mating et la conversion blanc-opaque : 1- c'est le locus MTL qui régule la faculté de C. albicans à effectuer le switch et 2- le mating des cellules en phase opaque est environ 10^6 fois plus efficace que celui des cellules en phase blanche. L'intérêt pour C. albicans de lier les 2 systèmes est peut-être de faire en sorte que les individus ne puissent se recombiner que dans des niches spécifiques. En effet, les cellules en phase opaque sont plus fragiles que les cellules en phase blanche et elles sont instables[2].
Le mating de deux cellules de C. albicans a pour résultat final une cellule tétraploïde, qui doit perdre certains de ses chromosomes pour rétablir la diploïdie.Chez la plupart des champignons, ce processus se fait grâce à une méiose qui complète ainsi un cycle sexuel. Toutefois, chez C. albicans, seul un cycle parasexuel a pu être identifié in vitro, un cycle impliquant une perte coordonnée des chromosomes surnuméraires au fil des divisions cellulaires[3]. Il reste toutefois possible que C. albicans puisse subir une méiose, puisque l'étude de son génome a permis d'identifier plusieurs orthologues de gènes impliqués dans ce processus chez d'autres levures : le locus MTL, NDT80, etc.[4] D'un autre côté, plusieurs gènes importants pour la méiose semblent manquer dans le génome de C. albicans, ce qui suggère que si la méiose peut avoir lieu dans cette levure, son déroulement doit être différent de celui des autres champignons.
C. albicans est un organisme diploïde qui possède huit paires de chromosomes, le plus grand étant appelé R, les suivants étant numérotés de 1 à 7 selon une taille décroissante. Son génome correspond approximativement à 16 Mb (haploïde) et code environ 6 400 gènes. Le code génétique de C. albicans possède une particularité. Le codon CUG code une sérine et non pour une leucine. Une des caractéristiques de C. albicans est sa très grande hétérozygotie naturelle, ce qui lui confère une grande capacité d'adaptation. Cette hétérozygotie accrue repose sur un réarrangement chromosomique (polymorphisme de longueur du chromosome), mais aussi des translocations réciproques, un polymorphisme nucléotidique ou encore des délétions de nucléotides. Ces modifications du caryotype entraîne des modifications à l'échelle phénotypique, ce qui peut ainsi se traduire par une grande adaptabilité de C. albicans.[5]
Il existe plusieurs techniques qui permettent d'identifier C. albicans en laboratoire, par exemple :
Un facteur de virulence permet à un pathogène de se maintenir et de proliférer dans son hôte. Il peut alors créer des lésions pathologiques.
Le dimorphisme correspond à la transition de la forme levure ellipsoïdale, qui se sépare des cellules filles après la cytokinèse, à la forme hyphale, dont les cellules filles restent liées les unes aux autres par des septa et dont la croissance est apicale. Cette transition morphologique réversible de forme levure à forme champignon[6] peut être induite par un grand nombre de stimuli : le pH, la température, la composition du milieu... Les voies de signalisation conduisant à la filamentation chez C. albicans sont soit MAP-kinase dépendante, soit pH-dépendante, soit AMPc-dépendante. Ces voies sont redondantes : le blocage de l'une d'elles ne suffit pas à inhiber la filamentation. D'autre part, les gènes régulés par ces voies (HWP1, ALS, SAP) sont connus pour leur rôle dans la virulence. Entre les formes levure et hyphale, on peut encore trouver d'autres formes morphologiques comme le pseudohyphe et la chlamydospore, qui sont toutefois plus rares[7].
C. albicans possède un grand nombre de récepteurs à sa surface qui lui permettent de reconnaître les cellules de son hôte et de s'y attacher solidement. Le β-1,2-phosphomannoside se lie ainsi à la galectine via une liaison lectinique. Les protéines de la famille ALS (agglutinin-like sequence) se lient à diverses protéines (laminine, collagène, fibrinogène) ainsi qu'à des cellules épithéliales et endothéliales via des liaisons non covalentes[8]. Enfin, la protéine Hwp1p (hyphal wall protein) se lie à son substrat de manière covalente par l'action d'une transglutaminase.
C. albicans possède toute une gamme d'enzymes hydrolytiques qui sont exprimées différentiellement selon l'environnement. On peut citer par exemple les enzymes de la famille SAP (secreted aspartyl proteinase), qui compte actuellement 10 membres et dont les rôles sont variés (dégradation de protéines, dégradation des structures cellulaires et tissulaires de l'hôte, dégradation du système immunitaire). Leur expression dépend du pH, de la localisation de C. albicans et de sa forme morphologique[9]. C. albicans possède encore des phospholipases (A, B, C et D) et des lipases (1 à 10).
Les antifongiques utilisés actuellement ont de nombreuses cibles : la paroi cellulaire, la membrane plasmique, la synthèse de l'ergostérol, l'ADN, l'ARN... Ces antifongiques sont soit fongistatiques, soit fongicides.
Les polyènes (ex. : Amphotéricine B [AmB], nystatine) sont des antifongiques naturels à action fongicide. L'AmB se lie à l'ergostérol de la membrane du champignon et crée des pores, augmentant ainsi la perméabilité de la membrane. Des composés essentiels à la vie du champignon diffusent ainsi hors du cytosol (ions K+...) ce qui conduit à la mort de l'organisme.
L'AmB connaît une réactivité croisée avec le cholestérol, le stérol principal chez l'homme. L'AmB peut donc être toxique à haute dose.
Les analogues de pyrimidine (ex. : 5-fluorocytosine [5-FC]) sont des antifongiques à action fongicide. La 5-FC pénètre la cellule fongique et inhibe la synthèse d'ARN et d'ADN. Les analogues de pyrimidine n'affectent pas l'homme, car la cytosine déaminase n'existe pas dans les cellules ou y est faiblement active.
Les azoles forment la classe la plus répandue d'antifongiques à action fongistatique. On les classe en 2 sous-familles : les imidazoles (kétoconazole, miconazole) et les triazoles, plus récents (fluconazole, voriconazole, itraconazole). Les azoles inhibent l'action du gène ERG11 impliqué dans la biosynthèse de l'ergostérol. La membrane est ainsi fragilisée et le champignon ne peut plus croître. Le gène ERG5 est une cible secondaire des azoles.
Les allylamines (ex. : terbinafine, naftinine) sont des antifongiques à action fongistatique qui inhibent la fonction de l'enzyme codée par ERG1, une squalène epoxidase. L'effet fongistatique est le résultat de la déplétion en ergostérol et de l'accumulation de stérols toxiques dans la cellule.
Les morpholines (ex. : amorolfine) ont une activité fongistatique en inhibant la fonction de 2 enzymes impliquées dans la biosynthèse de l'ergostérol : la C-14 stérol réductase (codée par ERG24) et la C-8 stérol isomérase (codée par ERG2).
La paroi cellulaire a une fonction importante chez les champignons. C'est pourquoi de nouveaux antifongiques ont été développés, qui ciblent la synthèse des éléments de ladite paroi. Les échinocandines ciblent par exemple le produit du gène FKS1 de C. albicans, qui code une β-1,3-glucane synthase. Les échinocandines (ex. : caspofongine, micafongine...) ont une forte activité fongicide et ne présentent pas de réactivité croisée avec d'autres antifongiques[10],[11],[12],[13],[14].
Parmi les autres types d'antifongiques, plus ou moins récents, on retrouve les sordarines, qui ciblent la synthèse de protéines (inhibiteurs du facteur d'élongation 2), l'auréobasidine A (inhibiteur de la synthèse de céramides), les inhibiteurs de pompes à protons ou des transporteurs ABC, etc.
La Ciclopiroxolamine fait partie de la famille des pyridones (dictionnaire Vidal 1996) et les candidoses cutanées font partie de ses indications thérapeutiques.
L'Horopito (Pseudowintera colorata), une plante qui pousse en Nouvelle-Zélande, est un antifongique naturel qui a une puissante action contre le C. albicans[10],[11],[12],[13],[14]. Cette plante est par ailleurs 32 fois plus efficace lorsqu'elle est associée à l'anis épicé d'Amérique du Sud (Pimpinella anisum)[15]. L'huile essentielle d'arbre à thé est également connue pour son efficacité contre Candida[16].
Les extraits de romarin (Rosmarinus officinalis) et de rhizome de gingembre (Zingiber officinale) sont aussi très efficaces contre toutes les souches de Candida courantes chez l'homme et ont été testés in vitro ainsi qu'in vivo chez la souris C57BL6. L'extrait éthanolique de gingembre est même plus efficace que le fluconazole sur Candida albicans, sa CMI (concentration minimale inhibitrice) est plus faible que l'antibiotique le plus souvent administré pour traiter les candidoses gynécologiques[17].
Certaines souches de C. albicans peuvent développer une résistance à la 5-FC en exprimant des formes mutées de cytosine perméase ou de cytosine déaminase. Mais la majorité des souches présentent des mutations dans le gène FUR1 qui code une phosphoribosyltransférase, empêchant ainsi à la 5-FC de s'intégrer dans l'ARNm lors de sa synthèse.
La résistance aux azoles est un phénomène courant chez C. albicans. Il intervient généralement lors de traitements prolongés avec le même médicament. La résistance peut intervenir de 4 manières[18] :
Cette absence d'Erg3p est couplée généralement à une résistance à l'AmB, puisque l'absence de la Δ5-6 désaturase coupe la voie de biosynthèse de l'ergostérol.
Les phénomènes de résistance aux échinocandines restent rares. On sait toutefois qu'une mutation dans le gène FKS1 suffit à rendre la souche résistante à l'action des échinocandines.
Candida albicans est l'espèce de levure la plus importante et la plus connue du genre Candida.
Candida albicans est un organisme vivant à l'état naturel dans les muqueuses de l'être humain. On le retrouve dans le tube digestif de 70% des adultes sains, et il n'entraîne habituellement aucune maladie ou symptôme en particulier. C'est un organisme commensal saprophyte.
Ce champignon pathogène provoque des infections fongiques (candidiase ou candidose) essentiellement au niveau des muqueuses digestive et gynécologique. Les candidoses sont une cause importante de mortalité chez les patients immunodéprimés comme les patients atteints du sida, les patients cancéreux sous chimiothérapie ou après transplantation de moelle osseuse. Les candidoses orale et œsophagienne sont fréquentes chez le patient atteint du sida. Lorsque Candida s'infiltre dans le flux sanguin, l'infection devient systémique et on parle alors de candidémie (ou candémie). Les candidémies sont caractérisées par une mortalité de l'ordre de 40 %. C. albicans peut donner également une multitude d'autres infections car il s'agit d'un pathogène opportuniste très polyvalent : il peut être responsable d'infection superficielle cutanée, causer un érythème fessier chez les nouveau-nés, une bronchopneumonie et, ou une pneumonie, une vaginite, une balanite ou être responsable d'infections profondes.
Au laboratoire médical, la culture en boîte de Petri des Candida donne des colonies qui sont grandes, rondes, de couleur blanche ou crème (albicans signifie « blanchâtre »).
Is fungas dioplóideach é Candida albicans (cineál ghiosta), a bhíonn i measc na n-orgánach a dhéanann atáirgeadh gnéasach, ach ní méóis. Is gníomhaire cúisíoch é, de réir mar a bhíonn caoi ann, in ionfhabhtuithe béalacha agus ginitiúla daonna.
Tá ionfhabhtuithe sistéamacha fungasacha (fungaimí) aitheanta mar údair tábhachtacha i ngalracht agus mbásmhaireacht na n-othar imdhíon-shochtaithe (m.sh. SEIF, ceimiteiripe ailse, galar fungasach sistéamach, trasphlandú smeara nó orgáin)
De bhreis ar sin, is ábhar mór imní anois iad na hionfhabhtuithe bainteach le hospidéal, faighte ag othair, nár cheapadh roimhe sin go mbeadh siad i mbaol. ( m.sh. othair a d'fhan san aonad dianchúram)
Candida albicans é unha especie de fungo diploide que pode crecer como un lévedo unicelular ou como fungo filamentoso multicelular, e é o axente causal de infeccións oportunistas orais e xenitais en humanos,[3][4] e da onicomicose candidal (infección na lámina ungueal da uña). As infeccións fúnxicas sistémicas por C. albicans (e outros fungos) son comúns en persoas inmunocomprometidas. Poden formarse biopelículas de C. albicans na superficie de aparellos médicos implantables. Ademais, as infeccións adquiridas en hospitais por C. albicans son un problema crecente.
C. albicans é un comensal e un constituínte da flora intestinal normal que comprende os microorganismos que viven na boca e no tracto gastrointestinal. C. albicans vive no 80% da poboación humana sen causarlle efectos nocivos, pero o sobrecrecemento do fungo dá lugar á candidíase. A candidíase é frecuente en individuos inmunocomprometidos, como os pacientes de SIDA ou os que foron sometidos a quimioterapia ou transplantes. Unha forma común de candidíase é a restrinxida ás membranas mucosas da boca ou vaxina, que se cura facilmente nas persoas cun sistema inmunitario en condicións normais. Informouse dunha elevada prevalencia da colonización por C. albicans en mozos con piercings na lingua, en comparación cos indivuduos que non os tiñan.[5] Para infectar os tecidos do hóspede, a forma usual unicelular de tipo lévedo de C. albicans reacciona a estímulos ambientais e cambia á forma invasiva multicelular filamentosa, un fenómeno chamado dimorfismo.[3]
Unha das características máis importantes do xenoma de C. albicans é a presenza de rearranxos cromosómicos numéricos e estruturais que serven como medio para xerar diversidade xenética, denominados polimorfismos de lonxitude (contracción/expansión de repeticións), translocacións recíprocas, delecións xenéticas e trisomía de cromosomas individuais. Estas alteracións cariotípicas producen cambios no fenotipo, que son unha estratexia de adaptación deste fungo. Estes mecanismos serán entendidos mellor cando se complete a análise do xenoma de C. albicans.
Unha característica pouco usual do xénero Candida é que en moitas das súas especies (incluíndo C. albicans e C. tropicalis, pero non, por exemplo, C. glabrata) o codón CUG, que normalmente especifica a leucina, nestas especies codifica a serina. Este é un raro exemplo dunha desviación do código xenético estándar, e a maioría desas desviacións están localizadas nos codóns de inicio ou, en eucariotas, nos códigos xenéticos mitocondriais.[6][7][8] En certos ambientes, esta alteración pode axudar a estas especies de Candida a inducir unha resposta de estrés permanente, unha forma máis xeneralizada de resposta ao shock térmico.[9]
O xenoma de C. albicans é moi dinámico, e esta variabilidade foi utilizada ventaxosamente para estudos epidemiolóxicos moleculares e estudos de poboación nesta especie. A secuenciación do xenoma permitiu a identificación da presenza dun ciclo parasexual (división meiótica ata entón non detectada) en C. albicans.[10] Este estudo da evolución da reprodución sexual en seis especies de Candida atopou perdas recentes nos compoñentes da principal vía de formación dos sobrecruzamentos meióticos, pero a conservación dunha vía menor.[10] Os autores suxiren que se as especies de Candida sofren meiose utilizan unha maquinaria reducida ou unha maquinaria diferente da normal, e indican que poden existir ciclos meióticos non coñecidos en moitas especies. Noutro estudo evolutivo, a introdución dunha redefinición parcial da identidade do cocón CUG desde especies de Candida a clons de Saccharomyces cerevisiae causaba unha resposta ao estrés que afectaba negativamente á reprodución sexual. Esta redifinición do significado de CUG, que apareceu nos antepasados de especies de Candida, crese que deixou pechadas a estas especies nun estado diploide ou poliploide cun posible bloqueo da reprodución sexual.[11]
Aínda que a miúido se fala de C. albicans como "dimórfica", de feito é polifénica. Cando se cultiva no medio estándar de laboratorio para lévedos, C. albicans crece como un "lévedo" de células ovoides. Porén, cambios ambientais suaves na temperatura e pH poden orixinar un cambio morfolóxico e as células pasan a un crecemento pseudohifal.[12] As pseudohifas comparten moitas semellanzas coas células de lévedos,[13] pero o seu papel durante a candidíase segue sendo descoñecido. Cando as células de C. albicans crecen nun medio que imita o ambiente fisiolóxico do corpo humano, crecen como hifas "verdadeiras". Propúxose que a súa capacidade de formar hifas é un factor de virulencia, xa que estas estruturas se observan frecuentemente nos tecidos invadidos, e as cepas que non poden formar hifas tampouco poden causar infeccións. C. albicans pode tamén formar clamidosporas, de función descoñecida.[14]
Nun proceso que superficialmente lembra o dimorfismo, C. albicans sofre un cambio fenotípico, no cal se xeran diferentes morfoloxías celulares espontaneamente. Entre as cepas normalmente estudadas, unha das que experimenta este cambio fenotípico é WO-1,[15] a cal presenta dúas fases: unha que crece formando células redondas e colonias brancas lisas, e outra con células de forma bacilar, que crece formando colonias grises planas. Outra cepa que se sabe que sofre cambio fenotípico é a 3153A; esta cepa produce polo menos sete morfoloxías de colonias diferentes. Tanto na cepa WO-1 coma na 3153A, cada fase convértese espontanemente na outra con baixa frecuencia. O cambio é reversible, e o tipo de colonias pode herdarse dunha xeración a outra. Identificaron varios xenes que se expresan de forma diferente en colonias con distintas morfoloxías, e algúns esforzos recentes céntranse en atopar que causa controla estes cambios. Ademais, unha tentadora cuestión é se hai ou non unha ligazón molecular entre o dimorfismo e o cambio fenotípico.[16]
Na cepa 3153A, atopouse un xene chamado SIR2 (ou "regulador da información silenciosa", do inglés silent information regulator), que parece ser importante para o cambio fenotípico. O SIR2 atopouse orixinalmente en Saccharomyces cerevisiae (lévedo de panadaría), no cal está implicado no silenciamento cromosómico, que é unha forma de regulación transcricional, na cal rexións do xenoma son inactivadas reversiblemente por cambios na estrutura da cromatina. Nos lévedos, os xenes implicados no control do tipo de apareamento están situados nestas rexións silenciadas, e SIR2 reprime a súa expresión ao manter nesa rexión unha estrutura da cromatina competente para o silenciamento. O descubrimento do C. albicans SIR2 implicado no cambio fenotípico tamén indica que ten rexións silenciadas controladas por SIR2, nas cales poden encontrarse os xenes específicos para o fenotipo.
Outra molécula potencialmente reguladora é Efg1p, que é un factor de transcrición que se encontra na cepa WO-1 e que regula o dimorfismo, e máis recentemente suxeriuse que axuda a regular o cambio fenotípico. A Efg1p exprésase só no tipo celular branco e non no gris, e a sobreexpresión de Efg1p na forma gris causa unha rápida conversión á forma branca.[17][18]
Ata agora, hai moi poucos datos que indiquen que o dimorfismo e o cambio fenotípico utilicen compoñentes moleculares comúns. Con todo, non é inconcibible que o cambio fenotípico poida ocorrer en resposta a algúns cambios no entorno. Saber como se regula SIR2 en S. cerevisiae pode proporcionar pistas de como funcionan os mecanismos para o cambio fenotípico en C. albicans.
A heterocigosidade do xenoma de Candida excede ao que se atopa noutros xenomas e está xeneralizada entre os illados clínicos. Os polimorfismos dunha soa base non sinónimos orixinan dúas proteínas que difiren nun ou en varios aminoácidos, o que produce diferenzas funcionais para cada proteína. Esta situación aumenta considerablemente o número de proteínas diferentes que codifica un xenoma.[19]
A Hwp1, que significa Proteína da Parede da Hifa 1 (do inglés Hyphal Wall protein 1) é unha manoproteína localizada na superficie das hifas da forma filamentosa de C. albicans. A Hwp1 é o substrato da transglutaminase de mamífero. Este encima do hóspede permite que C. albicans se una de forma estable ás células epiteliais do hóspede.[20] A adhesión de C. albicans a células hóspede é un primeiro paso esencial no proceso de infección para a colonización e a subseguinte indución da enfermidade nas mucosas.
Descubriuse recentemente que a proteína de unión ao ARN Slr1 xoga un papel en promover a formación de hifas e na virulencia de C. albicans.[21]
C. albicans foi utilizada en combinación con nanotubos de carbono para producir materiais de tecidos de bio-nano-composite condutores electricamente, que se utilizaron como elementos sensibles á temperatura.[22]
O tratamento xeralmente inclúe o uso de:[23]
Candida albicans é unha especie de fungo diploide que pode crecer como un lévedo unicelular ou como fungo filamentoso multicelular, e é o axente causal de infeccións oportunistas orais e xenitais en humanos, e da onicomicose candidal (infección na lámina ungueal da uña). As infeccións fúnxicas sistémicas por C. albicans (e outros fungos) son comúns en persoas inmunocomprometidas. Poden formarse biopelículas de C. albicans na superficie de aparellos médicos implantables. Ademais, as infeccións adquiridas en hospitais por C. albicans son un problema crecente.
C. albicans é un comensal e un constituínte da flora intestinal normal que comprende os microorganismos que viven na boca e no tracto gastrointestinal. C. albicans vive no 80% da poboación humana sen causarlle efectos nocivos, pero o sobrecrecemento do fungo dá lugar á candidíase. A candidíase é frecuente en individuos inmunocomprometidos, como os pacientes de SIDA ou os que foron sometidos a quimioterapia ou transplantes. Unha forma común de candidíase é a restrinxida ás membranas mucosas da boca ou vaxina, que se cura facilmente nas persoas cun sistema inmunitario en condicións normais. Informouse dunha elevada prevalencia da colonización por C. albicans en mozos con piercings na lingua, en comparación cos indivuduos que non os tiñan. Para infectar os tecidos do hóspede, a forma usual unicelular de tipo lévedo de C. albicans reacciona a estímulos ambientais e cambia á forma invasiva multicelular filamentosa, un fenómeno chamado dimorfismo.
Candida albicans adalah spesies cendawan patogen dari golongan deuteromycota. Spesies cendawan ini merupakan penyebab infeksi oportunistik yang disebut kandidiasis pada kulit, mukosa, dan organ dalam manusia.[3] Beberapa karakteristik dari spesies ini adalah berbentuk seperti telur (ovoid) atau sferis dengan diameter 3-5 µm dan dapat memproduksi pseudohifa.[3] Spesies C. albicans memiliki dua jenis morfologi, yaitu bentuk seperti khamir dan bentuk hifa. Selain itu, fenotipe atau penampakan mikroorganisme ini juga dapat berubah dari berwarna putih dan rata menjadi kerut tidak beraturan, berbentuk bintang, lingkaran, bentuk seperti topi, dan tidak tembus cahaya.[4] Cendawan ini memiliki kemampuan untuk menempel pada sel inang dan melakukan kolonisasi.[4]
Di dalam tubuh, Candida akan dikontrol oleh bakteri baik agar tetap berada dalam jumlah yang rendah dan seimbang. Bakteri baik dalam tubuh akan bekerja dengan cara memakan Candida. Sayangnya, antibiotik, pil pengontrol kehamilan, kortison, alkohol, sebagian besar makanan junk food, dan kemoterapi akan membunuh bakteri menguntungkan dalam tubuh (probiotik) sehingga menyebabkan jumlah Candida tidak terkendali. Saat pertumbuhannya berlebihan, Candida akan mengkolonisasi saluran pencernaan, berubah menjadi jamur, dan membentuk struktur seperti akar yang disebut rizoid. Struktur rizoid dapat menembus mukosa atau dinding usus, membuat lubang berukuran mikroskopik, dan menyebabkan racun, partikel makanan yang tidak tercerna, serta bakteri dan khamir dapat masuk ke alam aliran darah. Kondisi tersebut disebut sebagai sindrom kebocoran usus (leaky gut syndrome). Kebocoran pada dinding usus akan menyebabkan khamir seperti Candida dapat menyebar ke berbagai bagian tubuh, seperti mulut, sinus, tenggorokan, saluran reproduksi, jantung, dan kulit.[5]
Cendawan ini dapat memproduksi etanol (alkohol) yang memiliki efek intoksifikasi dalam darah bila kadarnya terlalu tinggi. Etanol tersebut dihasilkan dengan cepat ketika Candida memiliki sumber makanan berupa gula putih dan beberapa produk tepung lainnya. Di dalam kondisi yang akut, etanol diproduksi berlebihan hingga liver tidak dapat mengoksidasi dan mengeliminasinya. Selain itu, Candida juga dapat menyebabkan masalah menstrual dan hipotiroid. Candida dapat memproduksi hormon estrogen palsu sehingga tubuh menangkap sinyal bahwa produksi estrogen sudah mencukupi dan harus produksi hormon tersebut dihentikan. Masalah lainnya adalah pengiriman sinyal ke tiroid yang membuat produksi tiroksin dihentikan.[6]
Untuk mengatasi Candida, dapat dilakukan empat hal utama, yaitu membunuh khamir tersebut, mengurangi atau membatasi penggunaan antibiotik dan obat imunosupresif, diet atau pengurangan makanan yang dibutuhkan Candida untuk berkembang, menyembingkan dan meningkatkan sistem imun tubuh dengan penemenuhan kebutuhan nutrisi tubuh secara tepat.[7]
Salah satu cara terbaik untuk mengontrol Candida dalam tubuh melalui diet makanan adalah menghindari konsumsi segala jenis gula, tepung putih (white flour), minuman beralkohol, jamur, acar, makanan hasil fermentasi, kacang kering, keripik kentang, pretzel, junk food, bacon, daging babi hasil penggaraman, daging dan segala jenis keju.[6]
Candida albicans adalah spesies cendawan patogen dari golongan deuteromycota. Spesies cendawan ini merupakan penyebab infeksi oportunistik yang disebut kandidiasis pada kulit, mukosa, dan organ dalam manusia. Beberapa karakteristik dari spesies ini adalah berbentuk seperti telur (ovoid) atau sferis dengan diameter 3-5 µm dan dapat memproduksi pseudohifa. Spesies C. albicans memiliki dua jenis morfologi, yaitu bentuk seperti khamir dan bentuk hifa. Selain itu, fenotipe atau penampakan mikroorganisme ini juga dapat berubah dari berwarna putih dan rata menjadi kerut tidak beraturan, berbentuk bintang, lingkaran, bentuk seperti topi, dan tidak tembus cahaya. Cendawan ini memiliki kemampuan untuk menempel pada sel inang dan melakukan kolonisasi.
La Candida albicans[1] è un fungo saprofita. Si tratta di un lievito[2] appartenente alla famiglia dei Saccaromiceti.
Normalmente si trova nel cavo orale, nel tratto gastrointestinale e nella vagina.
Esso svolge un ruolo rilevante nella digestione degli zuccheri mediante un processo di fermentazione.
La Candida albicans è presente nell'organismo umano allo stato saprofitario, ma può diventare patogena in specifiche condizioni (opportunismo) provocando la candidosi. In particolare fra le altre specie di candida essa è l'unica che vive sulle mucose oltre che sulla cute.[3] La candida si moltiplica usualmente in modo anomalo nei fisici che hanno una qualche forma di squilibrio, un evento che in passato era marcatamente maggiore in soggetti debilitati e immunodepressi quali bambini, anziani e malati.[3]
Oggi queste forme di superinfezione di candida colpiscono anche soggetti sottoposti a lunghe cure antibiotiche (tetraciclina e neomicina, che intaccano la flora intestinale), stress prolungati e intensi o sbalzi ormonali. Il 60% delle donne che assumono la pillola soffre di candidosi vulvo-vaginale.
La candidosi si presenta quindi soprattutto come affezione vaginale (vaginite),[3] del cavo orale (mughetto) o della pelle. Attraverso l'intestino, può raggiungere il sangue dove libera le proprie tossine provocando la candidemia. Questo fenomeno dà luogo ad un corredo di sintomi quali gonfiore addominale, rallentamento della digestione, disturbi intestinali (stipsi o diarrea), intolleranze alimentari, stanchezza, irritabilità, insonnia, perdita di memoria, mal di testa e depressione. La candidosi induce anche un cattivo assorbimento delle sostanze nutritive e, a lungo andare, uno stato di malnutrizione.
La Candida albicans è un fungo saprofita. Si tratta di un lievito appartenente alla famiglia dei Saccaromiceti.
Normalmente si trova nel cavo orale, nel tratto gastrointestinale e nella vagina.
Esso svolge un ruolo rilevante nella digestione degli zuccheri mediante un processo di fermentazione.
Candida albicans is een gist die leeft in het maag-darmstelsel en de tractus urogenitalis. Het leeft daar als een zogenaamde commensaal en berokkent gewoonlijk geen schade.
Onder bepaalde omstandigheden, met name diegene waarbij het natuurlijke afweersysteem het laat afweten (bijvoorbeeld aids, inname van veel antibiotica, een ernstige ziekte) gaat deze gist soms 'overgroeien' en is er sprake van candidiasis. Het kan dan last veroorzaken in bijvoorbeeld de mond, maar ook ter hoogte van de genitalia. Candida albicans kan bij vrouwen zo leiden tot witte vloed. Bestrijding van Candida albicans is dan gewenst.
Candida albicans er en gjærsopp som ganske ofte kan gi infeksjon i munn og underliv (mest hos kvinner), sjeldnere infeksjoner i huden, spiserøret eller luftveiene. Sjelden og spesielt hos pasienter som er alvorlig syke av annen grunn, kan den gi sepsis ("blodforgiftning"). Slike infeksjoner kalles gjerne Opportunistiske infeksjoner, da de tar nytte av et nedsatt immunforsvar. Den trives i varme, fuktige omgivelser og har naturlig tilhold i munnen og fordøyelseskanalen. Mange får plager etter at de har brukt antibiotika for å behandle bakterie-infeksjoner. Grunnen til dette er at antibiotika ikke bare tar knekken på infeksjonen, men også andre mikroorganismer i kroppen. Dette gir Candida albicans bedre forhold. Områder med mye fuktighet og varme er mest utsatt, da spesielt området under brystene, hudfoldene i lysken og skjeden.
Også andre Candida-arter kan gi sykdom (C. glabrata, parapsilosis, tropicalis, krusei, dublinensis, norvegensis m.fl), men disse Pathogenene ses sjelden hos Immunkompetante (m.a.o ikke-Immunsvekkede) individer.
Candida-infeksjoner kan behandles med medikamenter enten lokalt (krem, vagitorier) eller med tabletter eller intravenøst. Tabletter i form av Flukanozol (Diflucan) gis ofte i en engangsdose i ukompliserte og mindre alvorlige tilfeller, slik som infeksjon i underlivet.
Candida albicans er en gjærsopp som ganske ofte kan gi infeksjon i munn og underliv (mest hos kvinner), sjeldnere infeksjoner i huden, spiserøret eller luftveiene. Sjelden og spesielt hos pasienter som er alvorlig syke av annen grunn, kan den gi sepsis ("blodforgiftning"). Slike infeksjoner kalles gjerne Opportunistiske infeksjoner, da de tar nytte av et nedsatt immunforsvar. Den trives i varme, fuktige omgivelser og har naturlig tilhold i munnen og fordøyelseskanalen. Mange får plager etter at de har brukt antibiotika for å behandle bakterie-infeksjoner. Grunnen til dette er at antibiotika ikke bare tar knekken på infeksjonen, men også andre mikroorganismer i kroppen. Dette gir Candida albicans bedre forhold. Områder med mye fuktighet og varme er mest utsatt, da spesielt området under brystene, hudfoldene i lysken og skjeden.
Også andre Candida-arter kan gi sykdom (C. glabrata, parapsilosis, tropicalis, krusei, dublinensis, norvegensis m.fl), men disse Pathogenene ses sjelden hos Immunkompetante (m.a.o ikke-Immunsvekkede) individer.
Candida-infeksjoner kan behandles med medikamenter enten lokalt (krem, vagitorier) eller med tabletter eller intravenøst. Tabletter i form av Flukanozol (Diflucan) gis ofte i en engangsdose i ukompliserte og mindre alvorlige tilfeller, slik som infeksjon i underlivet.
Bielnik biały (Candida albicans (C.P. Robin) Berkhout) – gatunek grzybów zaliczany do rzędu drożdżaków (Saccharomycetes)[1]. Jest to grzyb bezotoczkowy, wywołujący zakażenia oportunistyczne u chorych z obniżoną odpornością. Stanowi on florę fizjologiczną przewodu pokarmowego u 40-80% populacji.
Pozycja w klasyfikacji według Index Fungorum: Candida, Incertae sedis, Saccharomycetales, Saccharomycetidae, Saccharomycetes, Saccharomycotina, Ascomycota, Fungi[1].
Po raz pierwszy takson ten zdiagnozował w 1853 r. C. P. Robin nadając mu nazwę Oidium albicans. Obecną, uznaną przez Index Fungorum nazwę nadał mu w 1923 r. C. M Berkout, przenosząc go do rodzaju Candida[1].
Gatunek ten ma ponad 200 synonimów. Niektóre z nich: Candida stellatoidea (C.P. Jones & D.S. Martin) Langeron & Guerra i Oidium albicans C.P. Robin[2]:
Obecność 34 antygenów (zbudowanego głównie z mannanu) pozwala na różnicowanie w obrębie gatunku. Ze względu na różnicę jednego antygenu wyodrębniono C. albicans typ A (który posiada składnik antygenowy o numerze 6) od typu B, który tego składnika nie posiada[3].
Według analizy rRNA małej podjednostki rybosomu (16S), C. albicans razem z innymi przedstawicielami rodzaju Candida (C. tropicalis, C. parapsilosis i C. viswanathii) tworzy silnie wyodrębnioną od innych patogennych grzybów grupę[4].
C. albicans jest gatunkiem diploidalnym, posiada 8 par chromosomów. Genom C. albicans został w całości zsekwencjonowany w 2004 roku, liczy nieco ponad 14 milionów par zasad (wielkość haploidalnego genomu)[5].
Charakterystyczną cechą tego gatunku są często występujące rearanżacje chromosomowe (translokacje, delecje fragmentów chromosomów oraz aneuploidie) mogące prowadzić do zmian fenotypowych, co jest częścią strategii adaptacyjnej[6]. Zmiany w kariotypie występują często wśród izolatów klinicznych[7][8].
C. albicans korzysta z niestandardowego kodu genetycznego. Triplet CUG oznacza serynę, a nie leucynę, jak w standardowym kodzie genetycznym[9]. Podobna sytuacja ma miejsce w przypadku innych grzybów z rodzaju Candida (np. C. parapsilosis, C. dubliniensis, C. tropicalis, ale nie u C. glabrata[10][11]), a także m.in. u Debaryomyces hansenii, Pichia stipitis i Pichia farinsa. Gatunki te należą do jednostki systematycznej określanej mianem kladu CTG[12].
Podobnie jak w przypadku pozostałych grzybic, także tutaj infekcji sprzyja przewlekła antybiotykoterapia, osłabienie odporności, sztuczne zastawki, cewniki, zabiegi inwazyjne etc. Zazwyczaj kandydozę dzieli się na powierzchowną oraz znacznie poważniejszą uogólnioną. Obecność grzybów w moczu określa się mianem kandydurii.
W przypadku kandydozy powierzchownej biomateriał zanurzany jest w roztworze KOH, który niszczy keratynę nie wpływając na komórki grzyba. W preparacie mikroskopowym C. albicans widoczne są blastospory (pączkujące komórki), pseudostrzępki, czasami strzępki prawdziwe.
Grzyb wzrasta na pożywce Sabourauda w ciągu 2-4 dni, tworząc białe do kremowych kolonie, o masłowatej konsystencji. Brzeg kolonii jest gładki, czasami frędzlowaty ze względu na wytwarzaną grzybnię.
Wstępna identyfikacja opiera się na stwierdzeniu w hodowli na podłożu zubożonym (podłoże ryżowe) pod szkiełkiem nakrywkowym charakterystycznych zarodników przetrwalnikowych – chlamidospor. Drugą metodą jest wykonanie testu filamentacji (ang. germ tube test), który polega na inkubacji komórek C. albicans w surowicy przez 2-3 godziny w temperaturze 37 °C. Po tym czasie komórki „kiełkują” tworząc rostki, które w przeciwieństwie do tworzonych pączków lub pseudogrzybni nie są oddzielone ścianą komórkową. Powyższe testy nie pozwalają na różnicowanie C. albicans od C. dubliniensis dlatego potrzebne są dalsze testy najczęściej auksanograficzne.
Testy serologiczne wykorzystywane są do wstępnej diagnostyki zakażeń narządowych i uogólnionych oraz do monitorowania skuteczności leczenia. Nie pozwalają jednak na jednoznaczne określenie gatunku.
Diagnostyka oparta o metodę qPCR (reakcja łańcuchowa polimerazy w czasie rzeczywistym)
Metoda pozwala zidentyfikować poszczególne gatunki grzybów z rodzaju Candida (albicans, tropicalis, glabrata, krusei, para-ortho- metapsilosis) oraz określić ich liczebność, a w przypadku zakażeń mieszanych podaje odsetkowy udział poszczególnych gatunków grzybów w pobranej od pacjenta próbce.
Grzyb asymiluje glukozę, maltozę, sacharozę i galaktozę, ale nie laktozę. Dwa pierwsze węglowodany drobnoustrój fermentuje z wytworzeniem gazu, trzeci (galaktozę) tylko do kwasu. Fermentacja galaktozy jest zależna od szczepu. Grzyb nie wytwarza ureazy[3].
W przypadku zakażenia stosowane są[13] następujące leki przeciwgrzybicze:
Bielnik biały (Candida albicans (C.P. Robin) Berkhout) – gatunek grzybów zaliczany do rzędu drożdżaków (Saccharomycetes). Jest to grzyb bezotoczkowy, wywołujący zakażenia oportunistyczne u chorych z obniżoną odpornością. Stanowi on florę fizjologiczną przewodu pokarmowego u 40-80% populacji.
Candida albicans é uma espécie de fungo diplóide que causa, oportunamente, alguns tipos de infecção oral, peniana e vaginal nos seres humanos. As infecções causadas por fungos emergiram como uma das principais causas de morte em pacientes com algum tipo de imunodeficiência (como é o caso dos portadores da AIDS e das pessoas que estão passando por algum tipo de quimioterapia). Além disso, esse fungo pode ser perigoso para pacientes cuja saúde já esteja enfraquecida, como por exemplo os pacientes de uma unidade de tratamento intensivo. Devido a estes fatores, a Candida albicans tem despertado grande interesse das pesquisas na área de saúde e da medicina.
A Candida albicans está entre os muitos organismos que vivem na boca e no sistema digestivo humano. Sob circunstâncias normais, a Candida albicans pode ser encontrada em 80% da população humana sem que isso implique em quaisquer efeitos prejudiciais a sua saúde, embora o excesso resulte em uma infecção chamada Candidíase.
A virulência e patogenicidade da Candida albicans estão ligadas a diversos factores, sendo a formação de hifas, a estrutura da sua superfície celular (que, durante o contacto com células do hospedeiro, se adapta, sendo determinante para uma eficaz adesão e penetração), alterações fenotípicas (transição espontânea entre a forma típica de levedura, branca e circular, e uma forma opaca, em forma de pequenos bastões) e produção de enzimas extracelulares hidrolíticas os mais estudados ao longo dos últimos anos.
A patogenicidade da Candida albicans não pode ser atribuída a apenas um fator isolado; é da produção concomitante dos diversos factores que este organismo se transforma numa célula adaptada à invasão dos tecidos de um hospedeiro imunodeprimido. Como forma de resistência, tem capacidade de se multiplicar unicelularmente por gemulação. Na presença de compostos que induzem à sua patogenicidade, e.g. soro de mamíferos, a Candida albicans expressa os seus fatores de virulência, tal como a formação de hifas; estas capacitam a célula para exercer força mecânica, ajudando na sua penetração nas superfícies epiteliais, e uma vez na corrente sanguínea, têm uma ação danosa sobre o endotélio, o que permite que a Candida albicans invada os tecidos profundos do organismo (Kunamoto et al, 2005)(em casos mais graves, a invasão dos tecidos hepático e pancreático).
Em determinadas condições a Candida albicans desenvolve-se como uma pseudo-hifa, que se caracteriza por uma célula alongada que se propaga por uma gemulação unipolar, apresentando um aspecto de um cordão de contas.
Candida albicans é uma espécie de fungo diplóide que causa, oportunamente, alguns tipos de infecção oral, peniana e vaginal nos seres humanos. As infecções causadas por fungos emergiram como uma das principais causas de morte em pacientes com algum tipo de imunodeficiência (como é o caso dos portadores da AIDS e das pessoas que estão passando por algum tipo de quimioterapia). Além disso, esse fungo pode ser perigoso para pacientes cuja saúde já esteja enfraquecida, como por exemplo os pacientes de uma unidade de tratamento intensivo. Devido a estes fatores, a Candida albicans tem despertado grande interesse das pesquisas na área de saúde e da medicina.
A Candida albicans está entre os muitos organismos que vivem na boca e no sistema digestivo humano. Sob circunstâncias normais, a Candida albicans pode ser encontrada em 80% da população humana sem que isso implique em quaisquer efeitos prejudiciais a sua saúde, embora o excesso resulte em uma infecção chamada Candidíase.
Candida stellatoidea[1]
Oidium albicans[2]
Candida albicans [kándida álbikans] je vrsta kvasovk iz rodu Candida (kandida), ki najpogosteje povzroča kandidozo (okužba z eno od vrst kandid) pri ljudeh. Gre za oportunistično okužbo, kar pomeni, da se bolezen pojavi le pri osebah z zmanjšano odpornostjo. Okužba prizadene zlasti usta ali spolovila[3][4] Hujša oblika je sistemska kandidoza, ki je pogosto smrtna in prizadene predvsem bolnike s hujšo imunodeficienco (okrnjenost imunskega sistema) – bolniki z aidsom, zdravljene s kemoterapijo, po presaditvi kostnega mozga ...
C. albicans sicer naseljuje normalno črevesno floro in normalno ne povzroča škodljivih učinkov.
Candida albicans [kándida álbikans] je vrsta kvasovk iz rodu Candida (kandida), ki najpogosteje povzroča kandidozo (okužba z eno od vrst kandid) pri ljudeh. Gre za oportunistično okužbo, kar pomeni, da se bolezen pojavi le pri osebah z zmanjšano odpornostjo. Okužba prizadene zlasti usta ali spolovila Hujša oblika je sistemska kandidoza, ki je pogosto smrtna in prizadene predvsem bolnike s hujšo imunodeficienco (okrnjenost imunskega sistema) – bolniki z aidsom, zdravljene s kemoterapijo, po presaditvi kostnega mozga ...
C. albicans sicer naseljuje normalno črevesno floro in normalno ne povzroča škodljivih učinkov.
Candida albicans är en svampart. Denna svampsort liknar jäst och är vanligt förekommande. Den är en vanlig orsak till kronisk mukokutan kandidos, det vill säga en kronisk beläggning av svamp i kroppen. Man kan bära denna svampart utan att få några obehag, detta är mycket vanligt.
De nyttiga bakterierna lever i symbios i kroppen så ingen bakterie växer mer än de andra. När dessa bakterier samarbetar bildar de ett surt ämne som gör att Candida-svampen inte växer och blir för stor. På senare tid har man upptäckt att Candida-svampen lever i större skala och tar mer näring än bakterierna. Detta tros vara en koppling med antibiotika, då antibiotikan som ska döda de sjukdomsalstrande bakterierna även dödar många av de nyttiga bakterierna. Detta resulterar i att Candida-svampen kan växa och föröka sig.
En lösning till detta problem är att man kan inta mer probiotika-bakterier som är nyttiga bakterier och höjer immunförsvaret, samt hjälper till att återställa den naturliga balansen mellan Candida och de nyttiga bakterierna.[3]
Vid en infektion av Candida bör husläkare uppsökas om inte infektionen själv går över. Besvären finner man vanligast på huden, i munnen samt i slidan. Störst risk för infektion har spädbarn, gravida och äldre. I munhålan kallas denna infektion Torsk, och uppstår oftast om man använder tandprotes. Hos kvinnor är denna infektion vanligast i slidan och den är så vanlig att de flesta kvinnor har haft infektionen någon gång i livet. [4]
Candidainfektion kan även uppstå vid muntorrhet på grund av inhalation av exempelvis Symbicort, vilket beror på upplagring i munhålan, och kan motverkas av munsköljning efter inhalation. [5].
Man kan få tre symtom i munnen.
I kvinnans underliv är klåda och sveda de tydligaste symtomen. Vid sällsynta fall kan en vitaktig, luktfri flytning uppstå.
Det är svårt att förebygga sig mot en Candida-infektion eftersom den är så vanlig. Det man bör tänka på är att ha alla hudveck torra och rena. Ljumskar, hals, under brösten samt armar är några av de hudveck som är bra att hålla rena och torra. För kvinnor bör underkläder vara i bomullsmaterial.
Candida albicans är en svampart. Denna svampsort liknar jäst och är vanligt förekommande. Den är en vanlig orsak till kronisk mukokutan kandidos, det vill säga en kronisk beläggning av svamp i kroppen. Man kan bära denna svampart utan att få några obehag, detta är mycket vanligt.
Candida albicans, eşeyli çoğalan, diploit, maya tipi bir mantar türü ve insanlarda oral ve vajinal fırsatçı enfeksiyonların etmenidir. Candida cinsine ait 200 tür olmasına karşın Candida enfeksiyonlarının %75'inin sorumlusu C. albicans'tır. Türkçe okunuşu kandida albikanstır.
Bağışıklığı baskılanmış hastalarda (AIDS, kanser kemoterapisi, organ veya kemik iliği transferi durumlarında) sistemik mantarsal (fungal) enfeksiyonlar (fungemi), hastalık ve ölümün başlıca nedenleri arasındadırlar. Ayrıca bu yönde riski olmayan hastaların hastanede edindikleri enfeksiyonlar ciddi bir sağlık sorunu haline gelmiştir.
C. albicans insan ağzı ve sindirim sistemi içinde yaşayan pek çok organizmadan biridir. Sağlıklı yetişkinlerin %40'ının ağzında, sağlıklı kadınların %20-25'inin vajinasında varlığı gösterilebilir. C. albicans sindirim sistemindeki varlığıyla başka patojen bakterilerin çoğalmasını engeller. Vücudun bağışıklık sistemi ve diğer zararsız bakteriler normal şartlarda Candida'yı kontrol altında tutarlar.
Ancak, diğer bakterilerin sayısı C. albicans'a oranla azalırsa (örneğin antibiyotik kullanımından dolayı), bağışıklık sistemi zayıflamışsa veya mayanın çoğalmasına sağlayan başka şartlar mevcutsa (yüksek şeker, yüksek pH) C. albicans zararsız olan tek hücreli biçiminden, çok hücreli, istilacı (invasif), küf gibi ipliksi biçimine dönüşür (şekil [1]) ve vücudu istilaya başlar. C. albicansın iplikçi biçimi hem psödohif hem de gerçek hiflerden oluşabilir (şekil [2]). C. albicans iplikçi bir biçime dönüşmesine ilaveten, konak dokulara bağlanmasını sağlayan adhesinler, dokulara hem imha etmeye hem de onlara daha iyi yapışmayı sağlayan proteazlar, ve vücudun bağışıklık sisteminin tepkisini azaltan faktörler üretir.
C. albicans vücudun diğer çevreye açık ve nemli dokularında (ağız, vajina, bebeklerde alt bezi bölgesi) da aşırı çoğalırsa kandidoza yol açabilir. Kandidoz kanda ve genital yolda da oluşabilir. Ağızda C. albicans enfeksiyonuna pamukçuk denir.
Bağışıklığı baskılanmış hastalarda (örneğin HIV-pozitif hastalarda) Candida enfekziyonuna çok sık rastlanır ve bu hastalığın seyri çok daha ciddi olup fungemiye varabilir.
C. albicans genomunun en ilginç özelliklerinden biri genetik çeşitlilik yaratmak için kromozom uzunluk polimorfizmleri (dizin tekrarlarının daralması veya genişlemesi), karşılıklı translokasyonlar, kromozomal kaybolmalar (delesyonlar) ve belli kromozomlarda üçlenmeler (trizomiler) gibi yapısal değişikliklerin varlığıdır. Bu karyotipik değişiklikleri fenotip değişmelerine yol açtığı için bu organizmaya bir adaptasyon stratejisi sağlamaktadır. C. albicans genomunun analizi tamamlandığında bu değişikliklerin mekanizması daha iyi anlaşılacaktır.
Candida albicans genomunu Stanford DNA Dizileme ve Teknoloji Merkezi'nde (Stanford DNA Sequencing and Technology Center) dizilenmektedir. Bu proje için fonlar ABD Milli Diş ve Kranyofasyal Araştırma Kurumu (National Institute of Dental and Craniofacial Research) ve Burroughs-Wellcome Fonu (Burroughs-Wellcome Fund). tarafından sağlanmaktadır. Bir pilot dizileme projesi de Beowulf Genomics şirketi tarafından finanse edilip Sanger Center'de yürütülmektedir.
Çevresel şartlara bağlı olarak mantarların tek hücreli yapıdan iplikse yapıya geçebilmelerine çiftyapılılık (dimorfizm) denir. Çiftyapılılığa yüzeysel olarak benzeyen bir süreçte C. albicans fenotip sıçraması yoluyla farklı hücre şekillerine geçer. Fenotip sıçramasının klasik olarak çalışılmış olduğu suşlardan biri olan WO-1'de, iki evre vardır: biri parlak beyaz koloniler halinde büyür, öbürü ise çubuk şekillidir ve yassı gri koloniler olarak büyür. Sıçrama gösteren bir diğer suş olan 3153A ise en az yedi farklı koloni morfolojisi oluşturabilir (şekil [3]). Her iki suşta da farklı evreler birbirlerine düşük bir frekansla dönüşebilirler. Sıçrama geri tersinirdir ve koloni tipi kalıtsaldır. Farklı kolonilerde bazı genlerin farklı oranda ifade edildiği gösterilmiştir, ama biyologların asıl yoğun olarak araştırdıkları konu, bu değişikliklerin kontrol mekanizmalarıdır. Gen sıçramasına etki eden SIR2 geninin ipliksi büyümeye de etki ettiği gösterilmiştir, bu yüzden bu iki olgu birbiriyle ilişkilidir.
Candida albicans, eşeyli çoğalan, diploit, maya tipi bir mantar türü ve insanlarda oral ve vajinal fırsatçı enfeksiyonların etmenidir. Candida cinsine ait 200 tür olmasına karşın Candida enfeksiyonlarının %75'inin sorumlusu C. albicans'tır. Türkçe okunuşu kandida albikanstır.
Bağışıklığı baskılanmış hastalarda (AIDS, kanser kemoterapisi, organ veya kemik iliği transferi durumlarında) sistemik mantarsal (fungal) enfeksiyonlar (fungemi), hastalık ve ölümün başlıca nedenleri arasındadırlar. Ayrıca bu yönde riski olmayan hastaların hastanede edindikleri enfeksiyonlar ciddi bir sağlık sorunu haline gelmiştir.
C. albicans insan ağzı ve sindirim sistemi içinde yaşayan pek çok organizmadan biridir. Sağlıklı yetişkinlerin %40'ının ağzında, sağlıklı kadınların %20-25'inin vajinasında varlığı gösterilebilir. C. albicans sindirim sistemindeki varlığıyla başka patojen bakterilerin çoğalmasını engeller. Vücudun bağışıklık sistemi ve diğer zararsız bakteriler normal şartlarda Candida'yı kontrol altında tutarlar.
Ancak, diğer bakterilerin sayısı C. albicans'a oranla azalırsa (örneğin antibiyotik kullanımından dolayı), bağışıklık sistemi zayıflamışsa veya mayanın çoğalmasına sağlayan başka şartlar mevcutsa (yüksek şeker, yüksek pH) C. albicans zararsız olan tek hücreli biçiminden, çok hücreli, istilacı (invasif), küf gibi ipliksi biçimine dönüşür (şekil [1]) ve vücudu istilaya başlar. C. albicansın iplikçi biçimi hem psödohif hem de gerçek hiflerden oluşabilir (şekil [2]). C. albicans iplikçi bir biçime dönüşmesine ilaveten, konak dokulara bağlanmasını sağlayan adhesinler, dokulara hem imha etmeye hem de onlara daha iyi yapışmayı sağlayan proteazlar, ve vücudun bağışıklık sisteminin tepkisini azaltan faktörler üretir.
 Це незавершена стаття з мікології.
Це незавершена стаття з мікології.Candida albicans là một loài nấm có thể gây bệnh, nhưng lại là một thành phần thường gặp ở hệ vi sinh vật đường ruột của con người, hầu như không sinh sản và phát triển ngoài cơ thể con người. Trong các xét nghiệm thông thường, loài này đã được phát hiện trong đường tiêu hóa và miệng ở khoảng 40-60% người lớn khỏe mạnh.[4],[5]
Loài này được chú ý nhiều vì tuy thường là một sinh vật cộng sinh, nhưng lại có thể trở thành gây bệnh (bệnh cơ hội) ở những người bị suy giảm miễn dịch dưới nhiều điều kiện khác nhau, đặc biệt ở những bệnh nhân nhiễm HIV. Ngoài ra, nó còn gây viêm đường sinh dục cả ở nam và nữ, thậm chí đôi khi gây nguy hại tính mạng, nếu cơ hội cho phép nó phát triển quá mức. Tỷ lệ tử vong là 40% ở những người bệnh nhiễm nấm toàn thân toàn thân. Hàng năm, ước tính ở Hoa Kỳ có đến 2800 đến 11200 ca tử vong do nấm này. Thêm vào đó, Candida albicans là một loài sinh vật mô hình trong sinh học, đặc biệt là trong di truyền học phân tử (xem thêm ở trang Nhân tố chuyển vị ngược LTR).[6]
Mô tả sớm nhất về nấm này trong lịch sử thành văn của loài người thuộc về thầy thuốc Hippocrates - cách đây hơn 2.300 năm trong tác phẩm “In Epidemics”. Trong tài liệu này đã nhắc đến một dạng nấm “gây loét miệng”. Đến năm 1665, Pepys Diary đã báo cáo loại nấm này phát hiện ở “một bệnh nhân bị sốt, hay có cơn đau và bị nấc” do nấm ký sinh. Sau đó, các chuyên gia về Nấm học xác nhận. Đến cuối những năm 1900, Castellani xác định loại nấm này gây ra bệnh tưa miệng. Sau đó, Berg kết luận rõ ràng rằng nấm này còn gây ra viêm âm đạo, bệnh đường tiêu hóa.[7]
Tên khoa học theo nguyên tắc Linê bằng danh pháp hai phần là: Candida albicans, xuất phát từ chữ Latin "candeō" (candidus) trắng; còn "albicō" nghĩa là dần trở thành màu trắng. Thuật ngữ phân loại này được cho là của nhà sinh học Pháp là Charles-Philippe Robin (1821–1885) và nhà Nấm học Christine Marie Berkhout (1893–1932) đặt nên. Ngoài ra, còn nhiều tên gọi khác nữa.
Ngày nay, chi Candida của nấm này đã được xác định là có hơn 200 loài khác nhau. Trong bài này chỉ xét đến C. albicans và từ các mục dưới đây chỉ gọi tắt là nấm.
Nấm thuộc dạng vi nấm, kích thước rất nhỏ, mỗi cá thể trong suốt, bé nhất chỉ khoảng 5/1000 mm. Trong môi trường nuôi cấy nhân tạo (in vitro), nó biểu hiện rất nhiều kiểu hình khác nhau tuỳ theo chu kỳ sống và ảnh hưởng của các yếu tố môi trường. Ít nhất có đến bảy kiểu hình khác nhau được phát hiện, trong đó nó từ dạng như hình trứng (hình 2) có thể chuyển đổi thành dạng hình lưới (sợi nấm) có màu trắng đục (hình 3). Nếu gặp điều kiện bất lợi, nấm hoá thành dạng bào tử gọi là chlamydospores, có thể tồn tại rất lâu dài (hình 4). Ngược lại, nếu thuận lợi thì nấm có thể phát triển khá to (hình 5 và 6).
Nấm là đối tượng thuận lợi để nghiên cứu tái tổ gen trong quá trình gọi riêng là Candida albicans Retrotransposons (chuyển vị ngược của nấm này).[9]
Candida albicans là một loài nấm có thể gây bệnh, nhưng lại là một thành phần thường gặp ở hệ vi sinh vật đường ruột của con người, hầu như không sinh sản và phát triển ngoài cơ thể con người. Trong các xét nghiệm thông thường, loài này đã được phát hiện trong đường tiêu hóa và miệng ở khoảng 40-60% người lớn khỏe mạnh.,
Loài này được chú ý nhiều vì tuy thường là một sinh vật cộng sinh, nhưng lại có thể trở thành gây bệnh (bệnh cơ hội) ở những người bị suy giảm miễn dịch dưới nhiều điều kiện khác nhau, đặc biệt ở những bệnh nhân nhiễm HIV. Ngoài ra, nó còn gây viêm đường sinh dục cả ở nam và nữ, thậm chí đôi khi gây nguy hại tính mạng, nếu cơ hội cho phép nó phát triển quá mức. Tỷ lệ tử vong là 40% ở những người bệnh nhiễm nấm toàn thân toàn thân. Hàng năm, ước tính ở Hoa Kỳ có đến 2800 đến 11200 ca tử vong do nấm này. Thêm vào đó, Candida albicans là một loài sinh vật mô hình trong sinh học, đặc biệt là trong di truyền học phân tử (xem thêm ở trang Nhân tố chuyển vị ngược LTR).
Candida albicans (лат.) — диплоидный грибок (молочница) (форма дрожжеподобных грибов), способных к спариванию, но не в форме мейоза, возбудитель оппортунистических инфекций человека, которые передаются через рот и гениталии[1][2]. Систематические грибковые инфекции (фунгемии) являются важными причинами заболевания и смертности пациентов с иммунодефицитом (например, в результате СПИДа, химиотерапии рака или трансплантации органов). Кроме того, этот грибок является важным возбудителем инфекции, которые передаются в больницах.
Candida albicans один из организмов флоры кишечника, группы организмов, которых живут в человеческом рту и пищеводе. При нормальных обстоятельствах, C. albicans присутствует у 80 % людей, не вызывая болезней, хотя чрезвычайное увеличение его количества вызывает кандидоз. Кандидоз часто наблюдается у пациентов с иммунодефицитом, часто также поражает кровь и половые органы.
Candida albicans (лат.) — диплоидный грибок (молочница) (форма дрожжеподобных грибов), способных к спариванию, но не в форме мейоза, возбудитель оппортунистических инфекций человека, которые передаются через рот и гениталии. Систематические грибковые инфекции (фунгемии) являются важными причинами заболевания и смертности пациентов с иммунодефицитом (например, в результате СПИДа, химиотерапии рака или трансплантации органов). Кроме того, этот грибок является важным возбудителем инфекции, которые передаются в больницах.
Candida albicans один из организмов флоры кишечника, группы организмов, которых живут в человеческом рту и пищеводе. При нормальных обстоятельствах, C. albicans присутствует у 80 % людей, не вызывая болезней, хотя чрезвычайное увеличение его количества вызывает кандидоз. Кандидоз часто наблюдается у пациентов с иммунодефицитом, часто также поражает кровь и половые органы.
白色念珠菌(學名:Candida albicans)是一種能造成伺機性感染的酵母菌[5],常見於人類消化道與泌尿生殖道的菌群,約有四成至六成健康成人的口腔與消化道中都有白色念珠菌[6][7],平時與人體行片利共生,但可在人體免疫缺陷(如感染愛滋病[8])等特殊情況下過度生長[7][9]而造成念珠菌症[7][9],是念珠菌屬最常致病的菌種,與熱帶念珠菌(英语:Candida tropicalis)、副口炎念珠菌(英语:Candida parapsilosis)、光滑念珠菌(英语:Candida glabrata)合計造成了50%-90%的念珠菌症感染[9][10][11]。白色念珠菌也是最常在醫療器材或人體組織的生物薄膜中發現的真菌種類[12][13],這種真菌造成的系統性感染致死率高達40%[14]。据一个估计,在医院感染到的侵袭性念珠菌病在美国每年造成2,800至11,200人死亡[15]。
在生物學研究中,白色念珠菌常被當作模式生物,容易在實驗室中培養,並可以改變培養基成分的方式控制其生長型態,而用來進行不同主題的研究[16]。白色念珠菌屬於雙態性真菌,可以酵母型或菌絲型兩種方式生長,除此之外還有許多種不同的形態表型。長久以來,白色念珠菌被認為生活史中只存在雙倍體,而沒有單倍體,但後來單倍體與四倍體皆被發現[17],其雙倍體的基因組長度為29Mb[18]。
单倍体的白色念珠菌的基因组的大小約为16Mb(二倍体則为28Mb),由8條染色体对组成,分別称为chr1A,chr2A,chr3A,chr4A,chr5A,chr6A,chr7A和chrRA。二倍體時的第二组染色體具有相似的名称,但最后有一个字為B,如Chr1B,chr2B,...和chrRB。整个基因组包含6198个开放阅读框,这些ORF中有70%尚未被鉴定。白色念珠菌的基因组已經完成测序,为首批完全测序的真菌之一(与酿酒酵母和粟酒裂殖酵母(英语:Schizosaccharomyces pombe)相近)。除了開放閱讀框的資料庫外,還有GRACE(基因置换和条件表达)的資料庫,可用于研究白色念珠菌基因组中的必需基因[19][20]。研究白色念珠菌的最常用菌株是WO-1和SC5314菌株,WO-1菌株可高频率地在白色型與不透明型之间互相轉換,而SC5314菌株是用于基因定序参考標準的菌株[21]。
白色念珠菌的基因组最重要的特征之一是高度杂合性,由染色体長度多型性(重複序列的長度不一)、相互易位、染色體刪除、單核苷酸多態性與個別染色體的三倍體症等因素,造成染色體數量或結構上的重組,這些核型的改变會导致表現型的改变,是這種真菌對環境适应的機制。随着白色念珠菌基因组資訊的成功解碼,關於這些機制的研究正持續進行中[22][23][24]。
白色念珠菌(學名:Candida albicans)是一種能造成伺機性感染的酵母菌,常見於人類消化道與泌尿生殖道的菌群,約有四成至六成健康成人的口腔與消化道中都有白色念珠菌,平時與人體行片利共生,但可在人體免疫缺陷(如感染愛滋病)等特殊情況下過度生長而造成念珠菌症,是念珠菌屬最常致病的菌種,與熱帶念珠菌(英语:Candida tropicalis)、副口炎念珠菌(英语:Candida parapsilosis)、光滑念珠菌(英语:Candida glabrata)合計造成了50%-90%的念珠菌症感染。白色念珠菌也是最常在醫療器材或人體組織的生物薄膜中發現的真菌種類,這種真菌造成的系統性感染致死率高達40%。据一个估计,在医院感染到的侵袭性念珠菌病在美国每年造成2,800至11,200人死亡。
在生物學研究中,白色念珠菌常被當作模式生物,容易在實驗室中培養,並可以改變培養基成分的方式控制其生長型態,而用來進行不同主題的研究。白色念珠菌屬於雙態性真菌,可以酵母型或菌絲型兩種方式生長,除此之外還有許多種不同的形態表型。長久以來,白色念珠菌被認為生活史中只存在雙倍體,而沒有單倍體,但後來單倍體與四倍體皆被發現,其雙倍體的基因組長度為29Mb。